Abstract
Research Article
Severe brimonidine eye drop intoxication in a neonate as an accidental oral ingestion
Musa Silahli*
Published: 04 January, 2022 | Volume 5 - Issue 1 | Pages: 001-003
Brimonidine tartrate eye drops are a topical agent used to treat glaucoma in children over 2 years of age and adults. It is banned for children younger than 2 years of age because post-marketing studies have shown serious side effects. Colic is common in infants, which worries parents. And parents often use herbal and chemical medicines to solve this problem. We present a 12-day-old newborn with brimonidine eye drop intoxication, in which the drug was mistakenly administered orally to treat the colic problem.
Read Full Article HTML DOI: 10.29328/journal.japch.1001044 Cite this Article Read Full Article PDF
References
- Levy Y, Zadok D. Systemic side effects of ophthalmic drops. Clin Pediatr (Phila). 2004; 43: 99-101. PubMed: https://pubmed.ncbi.nlm.nih.gov/14968900/
- Gray C. Systemic toxicity with topical ophthalmic medications in children. Paediatr Perinat Drug Ther. 2006; 7: 23-29.
- Alphagan P. Irvine, CA: Allergan Incorporated; 2007
- Kiryazov K, Stefova M, Iotova V. Can ophthalmic drops cause central nervous system depression and cardiogenic shock in infants? Pediatr Emerg Care. 2013; 29: 1207-1209. PubMed: https://pubmed.ncbi.nlm.nih.gov/24196091/
- Al-Shahwan S, Al-Torbak AA, Turkmani S, et al. Side-Effect profile of Brimonidine Tartrate in Children. 2005; 12: 2143-2148. PubMed: https://pubmed.ncbi.nlm.nih.gov/16225927/
- Lai Becker M, Huntington N, Woolf AD. Brimonidine tartrate poisoning in children: frequency, trends, and use of naloxone as an antidote. Pediatrics. 2009; 123: 305-311. PubMed: https://pubmed.ncbi.nlm.nih.gov/19124581/
- Bowman RJC, Cope J, Nıschal KK. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan®) in children. Eye. 2004; 18: 24-26. PubMed: https://pubmed.ncbi.nlm.nih.gov/14707960/
- Ghaffari Z, Zakariaei Z, Ghazaeian M, Jafari R, Ezoddin N, et al. Adverse effects of brimonidine eye drop in children: A case series. J Clin Pharm Ther. 2021; 46: 1469-1472. PubMed: https://pubmed.ncbi.nlm.nih.gov/33626597/
- Deslandes G, Bouquié R, Boels D, Grégoire M, Grison-Hernando H, et al. Accidental poisoning with brimonidine eye drops in a 9-day-old infant. Toxicologie Analytique et Clinique. 2015; 27: 14-17.
Figures:
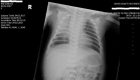
Figure 1
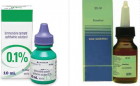
Figure 2
Similar Articles
-
Congenital poisoning after maternal parenteral mercury administrationBenjamin Courchia*,Leventer Roberts Maya,Meyer John,Galvez Maida,Herrera Jaime,Rauch Daniel. Congenital poisoning after maternal parenteral mercury administration. . 2018 doi: 10.29328/journal.japch.1001001; 1: 001-005
-
The Impact of Adenotonsillectomy on Health-Related Quality of Life in Paediatric PatientsShuaib Kayode Aremu*. The Impact of Adenotonsillectomy on Health-Related Quality of Life in Paediatric Patients. . 2018 doi: 10.29328/journal.japch.1001002; 1: 006-011
-
Severe Infantile Transaldolase deficiency: A case reportKhaled Alqoaer*,Ziad Asaad,Maisa Halabi. Severe Infantile Transaldolase deficiency: A case report. . 2019 doi: 10.29328/journal.japch.1001003; 2: 001-003
-
Prevalence of ESBL urinary tract infection in childrenKhalil Salameh*,Galia ZA Awean,Hala Elmohamed,Hoor Alshmayt,Mohamed Riad Bur Omer . Prevalence of ESBL urinary tract infection in children. . 2019 doi: 10.29328/journal.japch.1001004; 2: 004-007
-
Does it matter what a mother consumes? An anthropological exploration of dietary practices among Churachandpur (Manipur) pregnant women and its impact on infant birth weightJS Sehrawat*,Evelyn Ngaithianven,Reetinder Kaur. Does it matter what a mother consumes? An anthropological exploration of dietary practices among Churachandpur (Manipur) pregnant women and its impact on infant birth weight. . 2019 doi: 10.29328/journal.japch.1001005; 2: 008-014
-
Glycemic status and its effect in Neonatal Sepsis - A prospective study in a Tertiary Care Hospital in NepalBadri Kumar Gupta*,Binod Kumar Gupta,Amit Kumar Shrivastava,Pradeep Chetri. Glycemic status and its effect in Neonatal Sepsis - A prospective study in a Tertiary Care Hospital in Nepal. . 2019 doi: 10.29328/journal.japch.1001006; 2: 015-019
-
Branchio Oculo Facial SyndromeMaha Oudrhiri*,Toualouth L,Houda Oubejja,Fouad Ettayebi,Hicham Zerhouni. Branchio Oculo Facial Syndrome. . 2019 doi: 10.29328/journal.japch.1001007; 2: 020-020
-
Current childhood cancer survivor long-term follow-up practices in South AfricaAnel van Zyl*,Mariana Kruger,Paul C Rogers. Current childhood cancer survivor long-term follow-up practices in South Africa. . 2020 doi: 10.29328/journal.japch.1001008; 3: 001-007
-
Aripiprazole-induced seizures in children with autism spectrum disorder and epilepsyMohammed MS Jan*. Aripiprazole-induced seizures in children with autism spectrum disorder and epilepsy. . 2020 doi: 10.29328/journal.japch.1001009; 3: 008-010
-
Case-based education to improve learning and faculty teaching of residents and students in a clinical settingDiane E Lorant*,Elizabeth A Wetzel. Case-based education to improve learning and faculty teaching of residents and students in a clinical setting. . 2020 doi: 10.29328/journal.japch.1001010; 3: 011-015
Recently Viewed
-
Metastatic Brain Melanoma: A Rare Case with Review of LiteratureNeha Singh,Gaurav Raj,Akshay Kumar,Deepak Kumar Singh,Shivansh Dixit,Kaustubh Gupta*. Metastatic Brain Melanoma: A Rare Case with Review of Literature. J Radiol Oncol. 2025: doi: 10.29328/journal.jro.1001080; 9: 050-053
-
Validation of Prognostic Scores for Attempted Vaginal Delivery in Scar UterusMouiman Soukaina*,Mourran Oumaima,Etber Amina,Zeraidi Najia,Slaoui Aziz,Baydada Aziz. Validation of Prognostic Scores for Attempted Vaginal Delivery in Scar Uterus. Clin J Obstet Gynecol. 2025: doi: 10.29328/journal.cjog.1001185; 8: 023-029
-
Scientific Analysis of Eucharistic Miracles: Importance of a Standardization in EvaluationKelly Kearse*,Frank Ligaj. Scientific Analysis of Eucharistic Miracles: Importance of a Standardization in Evaluation. J Forensic Sci Res. 2024: doi: 10.29328/journal.jfsr.1001068; 8: 078-088
-
A study of coagulation profile in patients with cancer in a tertiary care hospitalGaurav Khichariya,Manjula K*,Subhashish Das,Kalyani R. A study of coagulation profile in patients with cancer in a tertiary care hospital. J Hematol Clin Res. 2021: doi: 10.29328/journal.jhcr.1001015; 5: 001-003
-
Additional Gold Recovery from Tailing Waste By Ion Exchange ResinsAshrapov UT*, Malikov Sh R, Erdanov MN, Mirzaev BB. Additional Gold Recovery from Tailing Waste By Ion Exchange Resins. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001098; 7: 132-138
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."