More Information
Submitted: June 22, 2023 | Approved: April 26, 2024 | Published: April 29, 2024
How to cite this article: Ashebir YG, Godie Y, Tadesse A, Mihretu E, Birhanu D. Prevalence and Associated Factors of Hypoglycemia among Severe Acute Malnourished Children who admitted in East Gojjam Zone Public Hospitals from 2018 to 2021, Northwest Ethiopia, 2022. Multi-center Retrospective Cross Sectional Study. J Adv Pediatr Child Health. 2024; 7: 037-044.
DOI: 10.29328/journal.japch.1001066
Copyright License: © 2024 Ashebir YG, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Severe acute malnutrition; Hypoglycemia; Under-five children; Ethiopia
Abbreviations: DMCSH: Debre Markos Compressive Specialized Hospital; MRN: Medical Registered Number; MG/DL: Milligram per Deciliter; RBS: Random Blood Sugar; RCT: Randomize Control Trial; SAM: Sever Acute Malnutrition; SC: Stabilize Centre; SPSS: Statistical Package for Social Science; WHO: World Health Organization
Prevalence and Associated Factors of Hypoglycemia among Severe Acute Malnourished Children who admitted in East Gojjam Zone Public Hospitals from 2018 to 2021, Northwest Ethiopia, 2022. Multi-center Retrospective Cross Sectional Study
Yitayal Guadie Ashebir1* , Yohannes Godie2, Aster Tadesse2, Esmelalem Mihretu2 and Dires Birhanu3
, Yohannes Godie2, Aster Tadesse2, Esmelalem Mihretu2 and Dires Birhanu3
1Department of Anesthesia, College of Health Science and Medicine, Debre Markos University, Debre Markos, Ethiopia
2Department of Pediatric and Child Health, College of Health Science and Medicine, Debre Markos University, Debre Markos, Ethiopia
3Department of Neonatal Nursing, Collage of Health Science and Medicine, Dilla University, Dilla, Ethiopia
*Address for Correspondence: Yitayal Guadie Ashebir, Department of Anesthesia, College of Health Science and Medicine, Debre Markos University, Debre Markos, Ethiopia, Email: [email protected]; [email protected]; [email protected]
Background: Globally, severe acute malnutrition (SAM) remains a major killer of children under 5 years of age. The highest magnitude is seen in sub-Saharan Africa, including Ethiopia. Hypoglycemia is the most common complication of severe acute malnutrition (SAM) and the most life-threatening condition in pediatric society. This study aimed to assess the prevalence of hypoglycemia and its associated factors among under-five children with severe acute malnutrition.
Methods: A cross-sectional retrospective study was conducted among 378 randomly selected samples who were admitted to public hospitals in the East Gojjam zone from 2018 to 2021. Data was extracted from the medical records of the children and entered into SPSS version 26, Variables with a p – value < 0.25 in the Bivariate analysis were candidates for multivariable logistic regression and those with a p – value < 0.05 in the multivariable analysis were considered as having a statistically significant association with hypoglycemia among severe acute malnutrition.
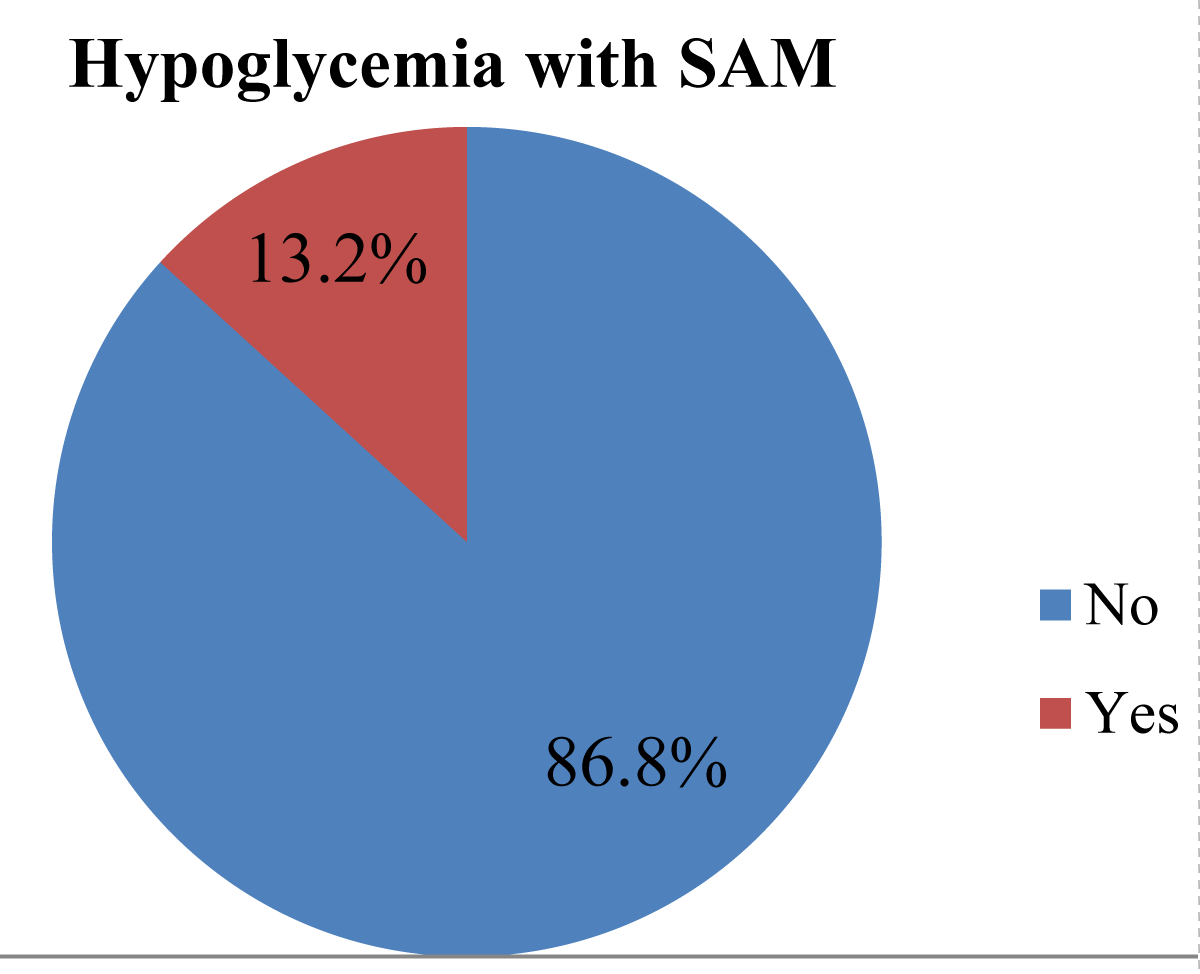
Results: Out of 378 respondents, 50 (13.2%) had hypoglycemia with severe acute malnutrition patients. Children admitted between the ages of 0-6 months were 2.93 (AOR = 1.57-6.25, p = 0.000), shocks were 4.6 (AOR = 1.25-17.42, p = 0.034), and fully immunized children were (AOR: 2.61 (1.01- 6.77, p = 0.048) was significantly associated with hypoglycemia with severe acute malnutrition.
Conclusion and recommendation: The prevalence of hypoglycemia with severe acute malnutrition was 13.2%. We also recommend a longitudinal study should be done among children who develop hypoglycemia with severe acute malnutrition to determine the long-term consequences, especially the neurodevelopmental sequelae associated with this condition.
At least one-third of all child fatalities worldwide are caused by malnutrition, which is a serious issue for global health. A state of abnormally low blood glucose levels is known as hypoglycemia [1]. The definition of hypoglycemia varies within the scientific literature and across clinical practice but is defined by the WHO as RBS < 2.5 mmol/L (45 mg/dL) in an adequately nourished child, or < 3.0 mmol/L (54 mg/dL) in a severely malnourished child [2]. Hypoglycemia is the most common complication of severe acute malnutrition (SAM) and a life-threatening threat to pediatric society. There is evidence that hypoglycemia is associated with higher mortality in children hospitalized with a severe illness [3]. Hypoglycemia has been found to have pro-inflammatory and prothrombotic effects and is associated with an increased risk of cardiovascular events [4,5]. It can also have severe neurological consequences, such as seizures and coma [2]. Globally, hypoglycemia with malnutrition increases the risk of morbidity and mortality, impairs cognitive development in children, and may also increase the risk of certain diseases throughout adulthood [6]. Worldwide trends show that malnutrition contributes to over half of all under-five child deaths due to hypoglycemia [7].
The hippocampus is particularly sensitive to hypoglycemia and can lead to deficits in cognitive development, particularly in working memory [8]. Severe acute malnutrition (SAM) is thought to predispose children to develop hypoglycemia [9]. Low glycogen stores and wasting with reduced lean mass and adipose tissue reserves have been linked to hypoglycemia in SAM [10]. In addition, hepatic glucose production is lower, especially in children with edematous SAM [11]. he WHO guidelines on the inpatient treatment of SAM at nutritional rehabilitation units recommend small, 2–3 hourly feeds in the early stages of treatment to prevent episodes of hypoglycemia [12].
It is believed that children with severe acute malnutrition (SAM) are more likely to experience either hypo or hyperglycemia. Low glycogen stores and wasting with reduced lean mass and adipose tissue reserves have been linked to hypoglycemia in SAM. Hormonal changes with impaired insulin responses as well as impaired glucose clearance could potentially increase the risk of hyperglycemia in SAM [4]. When compared to developed countries, hypoglycemia with SAM is a common health problem in developing countries [13]. This is evident when visiting almost any hospital in a developing country, where severely malnourished children and hypoglycemia are likely to account for a significant proportion of pediatric mortality [14]. Hypoglycemia with severe acute malnutrition is a common and serious condition that increases the mortality rate of children. Hypoglycemia in children with severe acute malnutrition can be easily treated and managed at a low cost, but there is a lack of awareness and materials in developing countries to assess hypoglycemia early in children with severe acute malnutrition. This study is intended to provide information and data on the prevalence of hypoglycemia among malnourished children admitted to health facilities. Such data is important for reassessing, evaluating, and reforming the policy. Reviewing previous studies on hypoglycemia in the malnourished yielded no clear and adequate data. The gap was addressed in this study. The goal of this study is to reduce morbidity and mortality associated with hypoglycemia caused by malnutrition and its risk factors.
Study design, area and period
A retrospective institutional-based cross-sectional study was conducted at public hospitals in the East Gojjam Zone from August 1 to August 30, 2022. This study was conducted at East Gojjam Zone public hospitals, Amara Region, Ethiopia. Debre Markos Town is the capital of the East Gojjam Zone, which is 254 kilometers away from Bahirdar, the capital of the Amhara National Regional State, and 304 kilometers away from Addis Ababa, the capital of Ethiopia. The study areas are Debre Markos Comprehensive Specialized Hospital, Lumamie Primary Hospital, and Bichena Primary Hospital. Debre Markos Comprehensive Specialized Hospital has a catchment area and five primary hospitals. The hospital provides care for around 5,000,000 of the population of Debre Markos town and its neighboring catchment area, and most of its patients come from lower socioeconomic areas; it admits 14 SAM patients per month [15]. Lumamie Primary Hospital provides care for 154,612 of the population of Lumama town and has 3 SAM patients per month admitted to it [16]. Bichena Primary Hospital provides care for around 522,000 of the population of Bichena town, including its catchment area, and has 5 SAM patients per month flow through it [17].
Eligible criteria and inclusion criteria
Under-five children admitted with SAM at the public hospital in East Gojjam Zone from 2018 to 2021 were included in this study. Children with incomplete data were excluded (i.e., outcome variable, age, sex, residence, routine medication, unknown admission and discharge date).
Sample size determination and Sampling technique
Sample size determination: The sample size was determined using the single population proportion formula. The following assumptions were made: that the marginal error (d) on either side of the true proportion was 5%, that a 95% confidence level was used, and that
Where n = minimum sample size required
D = Desired degree of error
z = standard normal distribution value at 95% confidence level
= 1.96 for 95% confidence intervalP = In an Ethiopian study of children with SAM, hypoglycemia was found to be the most common comorbidity and complication on admission, affecting 8.8% of children admitted to the stabilized center at the Gedo zone [18]. To maximize precision in our study, we used the lowest possible margin of error (d) of 3%, or 0.03.
Using a 10% non-response rate so, the final sample size was 378.
Sampling procedure and technique
Under-five children with SAM admitted to public hospitals in East Gojjam Zone from 2018 to 2021 were obtained. Each year, the number of admitted children was counted. The medical record was extracted within the defined period, and a unique number was assigned. The samples were proportionally allocated for each year. Each year, the final sample size was determined using a simple random sampling method.
Variable of the study
Dependent variable: Hypoglycemia with SAM.
Independent variable: Socio-demographic and admission status (age, sex, residence, history of TB contact and presence of edema, appetite test, admission criteria). Comorbidity (diarrhea, cough/pneumonia, malaria, sepsis, superficial infection (skin or ear infection), severe anemia, fever, HIV/AIDS, vomiting, hypothermia, hyperthermia).
Operational definition
Hypoglycemia: Occurs when the random blood sugar level falls below 54 mg/dL.
SAM: The presence of nutritional edema (bilateral pitting edema) or severe wasting (MUAC < 11.5 cm or a WFH < -3 z-score [WHO standards]) in children > 6 months old.
New admission: patients who are directly admitted to inpatient care to start the nutritional treatment.
Defaulted: SAM cases that are signed (by parents on behalf of their child) against treatment to leave treatment before cure or lost for 2 consecutive days with unknown status
Relapse: If that patient has ever been severely malnourished before and cured [19].
Marasmic-Kwash: SAM cases with both edema and severe wasting [19].
Data collection tool and procedure
Data collection has been done using a structured data extraction format accomplished by reviewing children’s medical record charts and the HMIS log book. After carefully observing the medical records of the children an appropriate data extraction checklist was made. The questionnaire was developed in English and used to collect data after being pre-tested before the study period. A modification of the questionnaire was made based on the pre-test. Socio-demographic characteristics, medical comorbidities, and treatment are given and follow-up characteristics of the study subject in the course of treatment of hypoglycemia with SAM. The data collection tools were adapted from several different related studies of hypoglycemia with SAM [20]. Four BSc nurses working in the malnutrition center collected the data by reviewing children’s medical record charts and the HMIS log book.
Data quality control
The data was extracted by staff nurses working in malnutrition centers who were familiar with the malnutrition follow-up form. Two days of introductions about the objectives, significance, and variables of the research, and how to extract the data by using the data extraction tools were given to four data collectors and two supervisors. To assess the consistency of the data extraction tools, a pretest was performed on approximately 5% of the charts at Dejen Primary Hospital. The data collection process was closely monitored by the supervisors and the principal investigator for its completeness and consistency.
Data analysis technique
Data was checked for completeness and consistency, then entered into SPSS and analyzed. SPSS 25 version statistical software was used for cleaning, coding, and analysis. Descriptive statistics were computed for categorical variables. The primary outcome variable was categorized as “0” for without hypoglycemia individuals and “1” for hypoglycemia with SAM individuals. The goodness-of-fit of the model was checked by the Hosmer–Lemeshow test. After checking the multi-collinearity and interaction terms, each independent variable with a p - value < 0.25 in the bivariate analysis was included in binary and multivariate logistic regression to control confounders. Finally, adjusted odds ratios (AOR) with p - values of 0.05 were used to determine statistical significance and measure the strength of the association. Finally, the result was presented in the form of text, tables, figures and a chart.
Socio-demographic characteristics
From 2018 to 2021, 378 under-five patients with severe acute malnutrition were admitted to East Gojjam zone Public Hospitals, with a 100% response rate. Out of 378 children, 240 were male (63.5%). In total, 308 (81.5%) of the respondents were aged 6-59 months; additionally, the majority of the children came from 250 rural (66.1%) homes, and half of the Marasmus respondents, 192 (50.8%), were 131 (34.7%). The 378 SAM children had a mean weight of 6.21 kg (SD = 4.61) and a mean height or length of 72.28 cm (SD = 13.8) at admission (Table 1).
| Table 1: Socio-demographic and admission information on enrolment of children with SAM treated by therapeutic center under-five children admitted East Gojjam Zone Public Hospitals from 2018 to 2021, Ethiopia, 2022 (n = 378). | |||
| Characteristics | Category | Frequency n = 378 | Percentage (%) |
| Sex | Male | 240 | 63.5 |
| Female | 138 | 36.5 | |
| Age | 0-6 month | 70 | 18.5 |
| 6-59 month | 308 | 81.5 | |
| Residence | Urban | 128 | 33.9 |
| Rural | 250 | 66.1 | |
| Clinical Classification of SAM |
Only edema (kwashiokor) | 131 | 34.7 |
| Only wasting (weight for height) or marasmus |
192 |
50.8 |
|
| Both edema & wasting (marasmus-kwashiokor) |
55 |
24.3 |
|
| Admission criteria | Bilateral pitting edema (any grade) |
92 | 24.3 |
| WFH<-3Z SCORE | 156 | 41.3 | |
| Severe wasting (MUAC < 11.5CM OR Z SCORE <-3) |
71 |
18.8 |
|
| Edema + severe Wasting + complication |
59 |
15.6 |
|
| Grade of nutritional edema |
no edema | 164 | 43.4 |
| Grade 1 or + | 106 | 28.0 | |
| Grade 2 or ++ | 74 | 19.6 | |
| Grade 3 or +++ | 34 | 9.0 | |
| MUAC at admission in mm |
<115 | 308 | 81.5 |
| Not indicated | 70 | 18.5 | |
| Admission status | New | 268 | 70.9 |
| Repeated | 43 | 11.4 | |
| Return after defaulter | 67 | 17.7 | |
| Appetite test at admission |
Pass | 48 | 12.7 |
| Fail | 330 | 87.3 | |
| Exclusive breast Feeding |
No | 139 | 36.8 |
| Yes | 239 | 63.2 | |
Medical comorbidities
There was no found hypoglycemia in 261 (69%) of the 378 SAM patients who presented, and 50 (13.2%) had found hypoglycemia with SAM. During admission, all but one of the respondents (346 out of 91.5) had comorbidity (Table 2).
| Table 2: Distribution of hypoglycemia and other medical comorbidities under five children with SAM admitted East Gojjam Zone Public Hospitals from 2018 to 2021, Ethiopia, 2022 (n = 378). | |||
| Characteristics | Category | Frequency n = 378 | Percentage (%) |
| Had the child comorbidities? | No | 32 | 8.5 |
| Yes | 346 | 91.5 | |
| Different comorbidity | No comorbidity | 32 | 8.5 |
| Hyperthermia (temp > 37.5 oc) | 20 | 5.3 | |
| Hypothermia (temp < 35.5 oc) | 100 | 26.5 | |
| Diarrhea | 90 | 23.8 | |
| Cough or pneumonia | 35 | 9.3 | |
| Malaria | 5 | 1.3 | |
| Vomiting | 62 | 16.4 | |
| Sepsis | 11 | 2.9 | |
| superficial infection(skin or ear) | 4 | 1.1 | |
| Severe anaemia | 10 | 2.6 | |
| HIV/AIDS | 3 | 0.8 | |
| Others | 6 | 1.6 | |
| Types of diarrhea | No diarrhoea | 302 | 79.9 |
| Watery diarrhoea | 60 | 15.9 | |
| Dysentery | 10 | 2.6 | |
| Other | 6 | 1.6 | |
| Degree of dehydration | no dehydration | 300 | 79.4 |
| some dehydration | 70 | 18.5 | |
| severe dehydration | 8 | 2.1 | |
| Level of consciousness | Normal | 105 | 27.8 |
| Altered | 273 | 72.2 | |
| Shock | No | 359 | 95.0 |
| Yes | 19 | 5.0 | |
| Failure to treatment | No | 335 | 88.6 |
| Yes | 43 | 11.4 | |
| TB | No | 368 | 97.4 |
| Yes | 10 | 2.6 | |
| Types of TB | has no TB | 368 | 97.4 |
| Pulmonary TB | 6 | 1.6 | |
| Other | 4 | 1.1 | |
Treatment is given and follow-up characteristics
East Gojjam Zone Public Hospitals used the national guideline for SAM Ethiopia inpatient treatment protocol for treatment given and follow-up characteristics of under-five children with SAM. Due to that, those under-five children admitted to the SAM room took different types of routine medication at admission, such as ampicillin and gentamicin (41.8%), amoxicillin (22.7%), and Albendazole or Mebendazole (8.7%) (Table 3).
| Table 3: Distribution of treatment given and follow-up characteristics under-five children with SAM admitted East Gojjam Zone Public Hospitals from 2018 to 2021, Ethiopia, 2022 (n = 378). | |||
| Characteristics | Category | Frequency | Percentage (%) |
| Has the child been given routine medication | No | 7 | 1.9 |
| Yes | 361 | 95.5 | |
| Not indicated | 10 | 2.6 | |
| Type of routine medication | not indicated for medication | 10 | 1.1 |
| Amoxicillin | 84 | 22.2 | |
| Ampicillin & Gentamicin | 158 | 41.8 | |
| Vitamin A | 5 | 1.3 | |
| Albendazole/mebendazole | 33 | 8.7 | |
| folic acid | 30 | 7.9 | |
| Anti-malaria | 3 | 0.8 | |
| measles vaccination | 24 | 6.3 | |
| Others | 37 | 9.8 | |
| child who had been given a zinc tab | No | 320 | 84.7 |
| Yes | 20 | 5.3 | |
| Not indicated | 38 | 10.1 | |
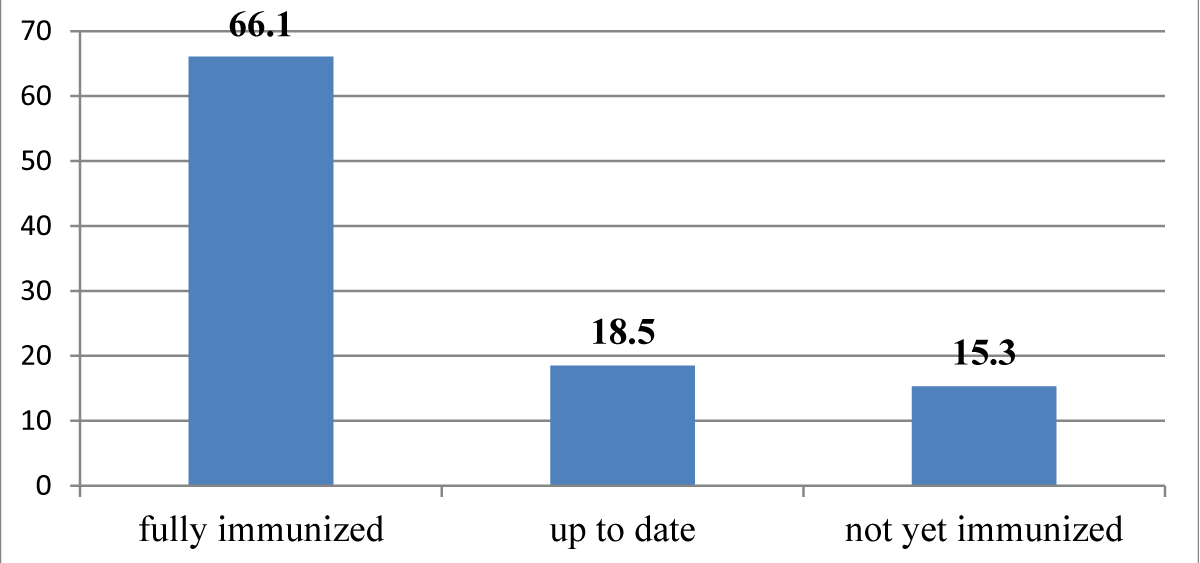
| Immunization status | fully immunized | 250 | 66.1 |
| up to date | 70 | 18.5 | |
| not yet immunized | 58 | 15.3 | |
| Child had fever | No | 20 | 5.3 |
| Yes | 358 | 94.5 | |
| The child was given special IV medication | No | 25 | 6.6 |
| Yes | 353 | 93.4 | |
| The child was given special IV medication | No | 0 | 0.0 |
| Yes | 353 | 93.4 | |
| Types of IV medication | had not taken special IV medication | 0 | 0.0 |
| Blood | 10 | 2.6 | |
| IV fluid | 70 | 18.5 | |
| IV anti-biotic | 293 | 77.5 | |
| Others | 5 | 1.3 | |
About 66.1% of under-five children were fully immunized (Figure 1).
Figure 1: Immunization status of under-five children with SAM admitted to east Gojjam zone public hospitals from 2018 to 2021, Ethiopia, 2022. (n = 378).
In terms of the prevalence of hypoglycemia among 378 patients studied, 50 (13.2%) were SAM patients (Figure 2).
Figure 2: Prevalence of hypoglycemia with severe acute malnutrition among under-five children who were admitted to East Gojjam public hospitals from 2018 to 2021, Ethiopia, 2022. (n = 378).
Factors associated with hypoglycemic among severe acute malnutrition
In the bivariate analysis, sex, age, residence, admission status, MUAC at admission, Appetite test at admission, shock, failure to treatment, TB, child who had given ReSoMal, type of IV medication, and immunization status were associated with significant variables. In contrast, age, shock, and children fully immunized were significantly associated with hypoglycemia among SAM in multivariate logistic regression analysis(p - value less than 0.05).
Age was significantly associated with hypoglycemia with SAM; children admitted between the ages of 0-6 months were 5.9 times more likely than those admitted between the ages of 6 and 59 months to develop hypoglycemia [AOR: 5.92 (2.41–14.52, p = 0.000)]. Children who had shock were 4.4 times more likely than those who did not have shock to develop hypoglycemia among SAM [AOR: 4.35 (1.12–16.91, p = 0.034)]. Children who had been fully immunized were 2.6 times more likely than those who had not yet been immunized to develop hypoglycemia among SAM (AOR: 2.61 (1.01- 6.77, p = 0.048) (Table 4).
| Table 4: Factors associated with hypoglycemia under five SAM children who were admitted to East Gojjam public hospitals from 2018 to 2021, Ethiopia, 2022 (n = 378). | |||||||
| Variable (n = 378) | Category | Hypoglycemia with SAM( n = 50) |
Binary regression | p -value | Multivariate regression | p -value | |
| Yes | No | COR (95%CI) | AOR (95%CI) | ||||
| Sex | Male Female |
143(59.8%) 118(84.9%) |
96(40.2%) 21(15.1%) |
3.77(2.22-6.42) 1 |
0.000 | 1.71(0.78-3.75) 1 |
0.185 |
| Age | 0-6 month 6-59 month |
21(30.0%) 240(77.9%) |
49(70.0%) 68(22.1%) |
8.24(4.62-14.68) 1 |
0.000 | 5.92(2.41-14.52) 1 |
0.000 |
| Residence | Urban-Rural | 77(60.2%) 184(73.6%) |
51(39.8%) 66(26.4%) |
1.85(1.18-2.90) 1 |
0.008 | 1.61(0.79-3.28) 1 |
0.193 |
| MUAC at admission in mm | <115 Not indicated |
233(75.6%) 28(40.0%) |
75(24.4% 42(60.0%) |
0.22(0.13-0.37) 1 |
0.000 | 0.69(0.25-1.87) 1 |
0.462 |
| Admission status | New Repeat Return P defaulter |
191(71.3%) 31(72.1%) 39(58.2%) |
77(28.7%) 12(27.9%) 28(41.8%) |
0.56(0.32-0.98) 0.54(0.24-1.23) 1 |
0.041 0.142 |
0.77(0.32-1.84) 0.71(0.21-2.36) 1 |
0.550 0.573 |
| Appetite test at admission | Pass Fail |
24(50.0%) 237(71.8%) |
24(50.0%) 93(28.2%) |
2.55(1.38-4.71) 1 |
0.003 | 0.72(0.28-1.87) 1 |
0.497 |
| Shock | Yes No |
256(71.3%) 5(26.3%) |
103(28.7%) 14(73.7%) |
6.96(2.44-19.82) 1 |
0.000 | 4.35(1.12-16.91) 1 |
0.034 |
| Immunization status | fully immunized up to date not yet immunized |
168(65.6%)
60(85.7%) 33(63.5%) |
88(34.4.4%)
10(14.3%) 19(36.5%) |
0.91(0.49-1.69)
0.29(0.12-0.69) 1 |
0.765 0.006 | 2.61(1.01- 6.77)
0.55(0.17-1.75) 1 |
0.048 0.313 |
| A child who had given ReSoMal | No Yes |
186(64.6%) 75(83.3%) |
102(35.4%) 152(16.7%) |
2.74 (1.49-5.02) 1 |
0.001 | 1.19(0.56-2.59) 1 |
0.644 |
| Failure to treatment | No Yes |
14(3.7%) 36(9.5%) |
86(22.8%) 242(64.0%) |
0.58 (0.30-1.12) 1 |
0.103 | 0.69(0.25-1.92) 1 |
0.480 |
| TB | No Yes |
252(68.5%) 9(90.0%) |
116(31.5%) 1(10.0%) |
4.14 (0.52-33.08) 1 |
0.180 | 2.14(0.24-18.72) 1 |
0.492 |
| Types of IV medication | Blood Iv fluid IV antibiotic Others |
8(80.0%) 52(74.3%) 200(68.3%) 1(20.0%) |
2(20.0%) 18(25.7%) 93(31.7%) 4(80.0%) |
0.06(0.00-0.92) 0.09(0.00-0.83) 0.12(0.01-1.05) 1 |
0.043 0.033 0.056 |
0.06(0.00- 1.15) 0.29(0.02-3.88) 0.13(0.01-1.58) 1 |
0.062 0.348 0.109 |
| Key: 1= Reference; * Statistically significant by COR at p - value ≤ 0.25; **Statistically significant by AOR at p - value < 0.05 | |||||||
In this study, a total of 378 sampled retrospective data records were reviewed, and the prevalence of hypoglycemia was 13.2% (95% CI: 0.1-0.17)). The study is lower than the study conducted in India (21.9%) [21]. Again, there was retrospective bias, and only 27% of patients had a blood glucose record in this system within 4 hours of admission WHO standards [22]. In our study, 50 of 378 (13.2%) SAM patients were admitted to three hospitals, including the East Gojjam Zone Public Hospital, for a retrospective study. A study conducted in Kenya examined the utility of the WHO protocol for a retrospective study of children with SAM and discovered a prevalence of hypoglycemia of 13.6% [23], This might be due to differences, in hospital facilities; skills of professionals, adherence to management protocol, the severity of cases, and comorbidity attributed to these variations.
The magnitude of hypoglycemia with SAM in our study was 13.2%, which is higher than the previous study in Ethiopia, Children with SAM who had hypoglycemia made up 8.8% of those admitted to the Gedo Zone [24]. The prevalence of hypoglycemia in SAM patients with comorbid pneumonia was 35 (9.3%) in this study, 14.3% in the previous study in Ethiopia [18] and in Malawi [25] 7 out of 176 (4%) SAM patients were recorded to have hypoglycemia in the first 24 hours of admission based on an electronic medical records database. The other study showed a retrospective analysis of case notes, relying on hypoglycemia being recognized and tested for; the prevalence of hypoglycemia was therefore likely underestimated based on observational data ranging from 1.3% to 31%. [25].
Children admitted between the ages of 0 and 6 months were 3 times more likely than those admitted between the ages of 6 and 59 months to develop hypoglycemia. This might be because the ages of 0 and 6 months of life are extremely important for the growth and development of children. It is a time when most malnutrition cases occur because it is a transition time from exclusive breastfeeding to complementary feeding.
Children who had shock were 5 times more likely than those who did not have shock to develop hypoglycemia among SAM. This finding was similar to previously conducted studies in Ethiopia Gedeon Zone [24] and Mozambique [20] of which shock was identified as the main determinant of death. This is because children with SAM are highly at risk of shock secondary to severe infections and diarrheal diseases, which can cause either hypovolemic or septic shocks. Besides, severe sepsis and diarrheal diseases in malnourished children might be associated with low cardiac reserves, leading to shock, which leads to death. Another possible explanation for the high fatality rate among children who got IV fluids is that the IV fluids prescribed to the children throughout the research period may not have adequately corrected their shock. This would be consistent with a study that compared the use of half-strength Darrow’s solution with the use of the isotonic solution in children with SAM and reported inadequate shock correction in all study arms, leading to a decision to end the trial early [4,5].
Children who had been fully immunized were 3 times more likely than those who had not yet been immunized to develop hypoglycemia among SAM. Malnourished children consequently stand to gain a great deal from vaccination, but given that it is the most prevalent immunodeficiency in the world, they may not be able to respond to immunizations as well as they could. Given that recurrent infections contribute to the pathophysiology of the illness, immunization may be essential for preventing malnutrition. The 10 greatest evidence-based nutrition-specific interventions are thought to minimize stunting by just 20%, highlighting the necessity for additional strategies, including infection control, to prevent growth failure in infancy. However, it is difficult to pinpoint the specific contribution of vaccination. Multi-sectoral interventions have proven the most successful in lowering the prevalence of stunting. Immunizations against one disease are unlikely to have a large impact on growth; rather, it is becoming more and more obvious that a combination of therapies is required to combat malnutrition [4].
This study revealed that children with SAM are at risk of hypo- or hyperglycemia, and hypoglycemia relates to clinical outcomes like mortality. Emphasize screening hypoglycemia in SAM patients about treating and preventing hypoglycemia. As this is a hospital-based secondary data analysis, further prospective studies are needed to identify the prevalence of hypoglycemia and risk factors for severe acute malnutrition in children.
The prevalence of hypoglycemia with severe acute malnutrition was 13.2%. We also recommend a longitudinal study should be done among children who develop hypoglycemia with severe acute malnutrition to determine the long-term consequences, especially the neurodevelopmental sequelae associated with this condition
Availability of data and materials
The data and other documents used in this study are available from the corresponding author.
Author’s contribution
YG, AT, YG, EM, and DB: conceptualization, methodology, data entry, data cleaning, data analysis, writing the original draft, validation, tool evaluation, methodology, reviewing, and editing. Finally, all authors approved the manuscript.
We would like to thank Debre Markos University, the College of Health Science, and the Department of Pediatrics and Child Health Nursing for their support in the preparation of this study. Hospital employees who allow any data collection from the hospital recorded review. Finally, thank you, data collectors and supervisors.
Ethical consideration
The study was conducted after receiving ethical approval from the research and ethics committee of the Debre Markos University Department of Pediatrics and Child Health Nursing. Before actual data collection, permission was taken from Debre Markos Referral Hospital, Lumamie Primary Hospital, and Bichena Primary Hospital’s chief executive officer. In addition to that, written informed consent was obtained from the study participants. Finally, the outcome was communicated to the clinician so that the patient could be treated. They were given the chance to ask any questions about the study and were free to refuse or stop the questionnaire at any time they wanted.
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R; Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013 Aug 3;382(9890):427-451. doi: 10.1016/S0140-6736(13)60937-X. Epub 2013 Jun 6. Erratum in: Lancet. 2013. 2013 Aug 3;382(9890):396. PMID: 23746772.
- Organization WH. Guideline: Updates on Paediatric Emergency Triage, Assessment, and Treatment: Care of Critically-Ill Children. World Health Organization Geneva. 2016.
- UNICEF/WHO/. World Bank Levels and Trends in Child Malnutrition – UNICEF WHO the World Bank Joint Child Malnutrition Estimates, key findings of the 2019 edition UNICEF. 2019.
- Ledger E, Harawa PP, Daniel AI, Candler T, Prentice AM, Bandsma RHJ. Dysglycemia in Children with Severe Acute Malnutrition: A Systematic Review and Meta-Analysis. Adv Nutr. 2021 Jun 1;12(3):959-968. doi: 10.1093/advances/nmaa138. PMID: 33179024; PMCID: PMC8166557.
- Bitew ZW, Ayele EG, Worku T, Alebel A, Alemu A, Worku F, Yesuf A. Determinants of mortality among under-five children admitted with severe acute malnutrition in Addis Ababa, Ethiopia. Nutr J. 2021 Dec 20;20(1):94. doi: 10.1186/s12937-021-00750-0. PMID: 34930311; PMCID: PMC8691009.
- Spoelstra MN, Mari A, Mendel M, Senga E, van Rheenen P, van Dijk TH, Reijngoud DJ, Zegers RG, Heikens GT, Bandsma RH. Kwashiorkor and marasmus are both associated with impaired glucose clearance related to pancreatic β-cell dysfunction. Metabolism. 2012 Sep;61(9):1224-30. doi: 10.1016/j.metabol.2012.01.019. Epub 2012 Mar 3. PMID: 22386944.
- Roberfroid D, Verstraeten R. Management of oedematous malnutrition in infants and children aged >6 months: a systematic review of the evidence. World Health Organization. 2013.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998 Jul;15(7):539-53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. PMID: 9686693.
- Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006 Jul;118(1):173-9. doi: 10.1542/peds.2005-1819. PMID: 16818563.
- Faustino EVS, Hirshberg EL, Asaro LA, Biagas KV, Pinto N, Srinivasan V, Bagdure DN, Steil GM, Coughlin-Wells K, Wypij D, Nadkarni VM, Agus MSD, Mourani PM, Chima R, Thomas NJ, Li S, Pinto A, Newth C, Hassinger A, Bysani K, Rehder KJ, Kandil S, Wintergerst K, Schwarz A, Marsillio L, Cvijanovich N, Pham N, Quasney M, Flori H, Federman M, Nett S, Viteri S, Schneider J, Medar S, Sapru A, McQuillen P, Babbitt C, Lin JC, Jouvet P, Yanay O, Allen C; Heart And Lung Failure-Pediatric INsulin Titration (HALF-PINT) Study Investigators. Short-Term Adverse Outcomes Associated With Hypoglycemia in Critically Ill Children. Crit Care Med. 2019 May;47(5):706-714. doi: 10.1097/CCM.0000000000003699. PMID: 30789401.
- Ratter JM, Rooijackers HM, Tack CJ, Hijmans AG, Netea MG, de Galan BE, Stienstra R. Proinflammatory Effects of Hypoglycemia in Humans With or Without Diabetes. Diabetes. 2017 Apr;66(4):1052-1061. doi: 10.2337/db16-1091. Epub 2017 Jan 23. PMID: 28115398.
- Mahajan G, Mukhopadhyay K, Attri S, Kumar P. Neurodevelopmental Outcome of Asymptomatic Hypoglycemia Compared With Symptomatic Hypoglycemia and Euglycemia in High-Risk Neonates. Pediatr Neurol. 2017 Sep;74:74-79. doi: 10.1016/j.pediatrneurol.2017.05.028. Epub 2017 Jun 7. PMID: 28739364.
- GOMEZ F, GALVAN RR, CRAVIOTO J, FRENK S. Malnutrition in infancy and childhood, with special reference to kwashiorkor. Adv Pediatr. 1955;7:131-69. PMID: 14349775.
- Waterlow JC. Classification and definition of protein-calorie malnutrition. Br Med J. 1972 Sep 2;3(5826):566-9. doi: 10.1136/bmj.3.5826.566. PMID: 4627051; PMCID: PMC1785878.
- Debremarkos Compressive Specialized Hospital. undrefive SAM patient IPD LOG registration book. 2022.
- Lumama primary hospital. Under five SAM patient ITP registration books. 2022.
- Bichena Primary Hospital. under five SAM patient ITP registration book. 2022.
- Ayanssa AB, Hailemariam TW, Melke AS. Determinants of acute malnutrition among children aged 6–59 months in Public Hospitals, Oromia region, West Ethiopia. 2017.
- Federal Ministry of Health. Protocol for the management of severe acute malnutrition Addis Ababa. 2014.
- Madrid L, Acacio S, Nhampossa T, Lanaspa M, Sitoe A, Maculuve SA, Mucavele H, Quintó L, Sigaúque B, Bassat Q. Hypoglycemia and Risk Factors for Death in 13 Years of Pediatric Admissions in Mozambique. Am J Trop Med Hyg. 2016 Jan;94(1):218-26. doi: 10.4269/ajtmh.15-0475. Epub 2015 Oct 26. PMID: 26503282; PMCID: PMC4710433.
- Samya V, Shriraam V, Jasmine A, Akila GV, Anitha Rani M, Durai V, Gayathri T, Mahadevan S. Prevalence of Hypoglycemia Among Patients With Type 2 Diabetes Mellitus in a Rural Health Center in South India. J Prim Care Community Health. 2019 Jan-Dec;10:2150132719880638. doi: 10.1177/2150132719880638. PMID: 31631765; PMCID: PMC6804359.
- Maitland K, Berkley JA, Shebbe M, Peshu N, English M, Newton C. Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol. PLoS Med. 2016; 3(500).
- Abhinay A, Kumar D, Rao SK. Triage of children with severe acute malnutrition and its outcome: single centre cross-sectional study. JCDR 2019; 13:4-6. DOI:10.7860/JCDR/2019/41044.12856
- Girum T, Kote M, Tariku B, Bekele H. Survival status and predictors of mortality among severely acute malnourished children <5 years of age admitted to stabilization centers in Gedeo Zone: a retrospective cohort study. Ther Clin Risk Manag. 2017 Jan 23;13:101-110. doi: 10.2147/TCRM.S119826. PMID: 28176953; PMCID: PMC5271381.
- Vonasek BJ, Chiume M, Crouse HL, Mhango S, Kondwani A, Ciccone EJ, Kazembe PN, Gaven W, Fitzgerald E. Risk factors for mortality and management of children with complicated severe acute malnutrition at a tertiary referral hospital in Malawi. Paediatr Int Child Health. 2020 Aug;40(3):148-157. doi: 10.1080/20469047.2020.1747003. Epub 2020 Apr 3. PMID: 32242509.