More Information
Submitted: July 18, 2023 | Approved: May 01, 2024 | Published: May 02, 2024
How to cite this article: Balyorugulu GG, Yusuph S, Majaliwa R, Innocent M, Martine F, et al. Case Report: An Elusive Case of Septic Arthritis. J Adv Pediatr Child Health. 2024; 7: 045-051.
DOI: 10.29328/journal.japch.1001067
Copyright License: © 2024 Balyorugulu GG, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Antibiotics; Culture; Fastidious organisms; Pediatric
Case Report: An Elusive Case of Septic Arthritis
Georgina George Balyorugulu1*, Shabani Yusuph2, Rahma Majaliwa1, Mpuya Innocent3, Fikiri Martine4, Fatma Said5, Rogatus Kabyemera6, Patrick Ngoya7 and Jeremiah Seni8
1Department of Pediatrics and Child Health, Kamanga Medics Hospital, Mwanza Tanzania
2Laboratory Department, Kamanga Medics Hospital, Mwanza Tanzania
3Department of General Surgery, Kamanga Medics Hospital, Mwanza Tanzania
4Department of Orthopedics and Trauma, Kamanga Medics Hospital, Mwanza Tanzania
5Department of Anaesthesiology, Kamanga Medics Hospital, Mwanza Tanzania
6Department of Pediatrics and Child Health, Bugando Medical Centre, Mwanza Tanzania
7Department of Radiology, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
8Department of Microbiology and Infectious Diseases, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
*Address for Correspondence: Georgina George Balyorugulu, Kamanga Medics Hospital, P.o.Box 5228, Mwanza, Tanzania, Email: [email protected]
Septic arthritis is a serious inflammatory infectious state of the joint secondary to microbial infection. In the pediatric population the most common route of infection is haematogenous spread. Less than fifty percent of patients with septic arthritis will yield positive culture results with a mortality rate of up to 42% in some cases. Due to the challenge in obtaining culture and identification of the causative organism the management of septic arthritis has been more of empirical in nature with the chosen antibiotic synchronized with the epidemiological data. Here is a case of a 14 months old female patient presenting at our hospital with a left knee and lower thigh swelling for three days with failure to bare weight on the limb. In addition, she had fever and diarrhea for three days. Upon evaluation clinical, laboratory and radiological findings supported septic arthritis expect for her blood, pus and synovial fluid culture of which all came back negative. She had poor response to intravenous ceftriaxone, gentamycin, metronidazole, ampicillin- cloxacillin and amoxicillin clavunate. Over the course of therapy, she developed septic shock, severe anemia and acute liver failure and was admitted to the intensive care unit. Afterwards she was initiated vancomycin and developed a hypersensitivity reaction with generalized edema which prompted cessation of the drug. Due to her critical state and poor response a triple therapy regimen composing of meropenem, ciprofloxacin and metronidazole was selected and maintained for three weeks followed by an oral clindamycin course for another three weeks of which she responded. In addition, surgical debridement arthrotomy, irrigation and drainage were done. Physiotherapy for rehabilitation is ongoing with patient recovering well.
Acute onset septic arthritis occurs in 4 to 5 cases per 100,000 with data ranging from 1/5000 in children aged less than five years to 1/20,000 in some African countries [1-3]. In one study, only 64% of the children with pyogenic arthritis had the etiological organism identified with Haemophilus influenza type B found in 82% of children aged 6-24 months [4]. Staphylococcus aureus is still the most common organism in septic arthritis together with other organisms such as Enterobacter and group A streptococcus [5]. Kingella kingae has been known to cause septic arthritis in children and more commonly than anticipated with suspicion being high in cases that present atypically [6,7]. In certain diseases such as sickle cell disease, salmonella has been identified as the common agent [8] while in countries such as Kenya, Zambia and Malawi salmonella has been identified as a common agent in infant septic arthritis [9-11].
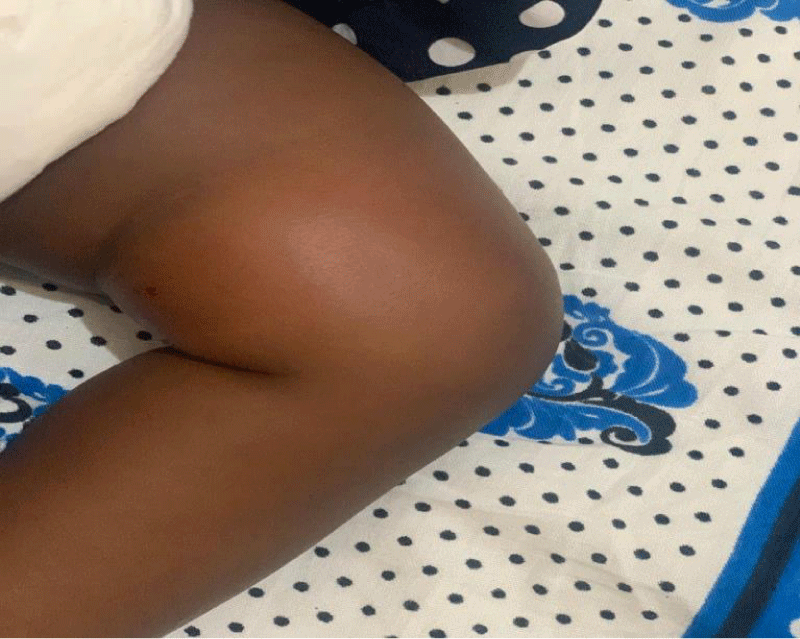
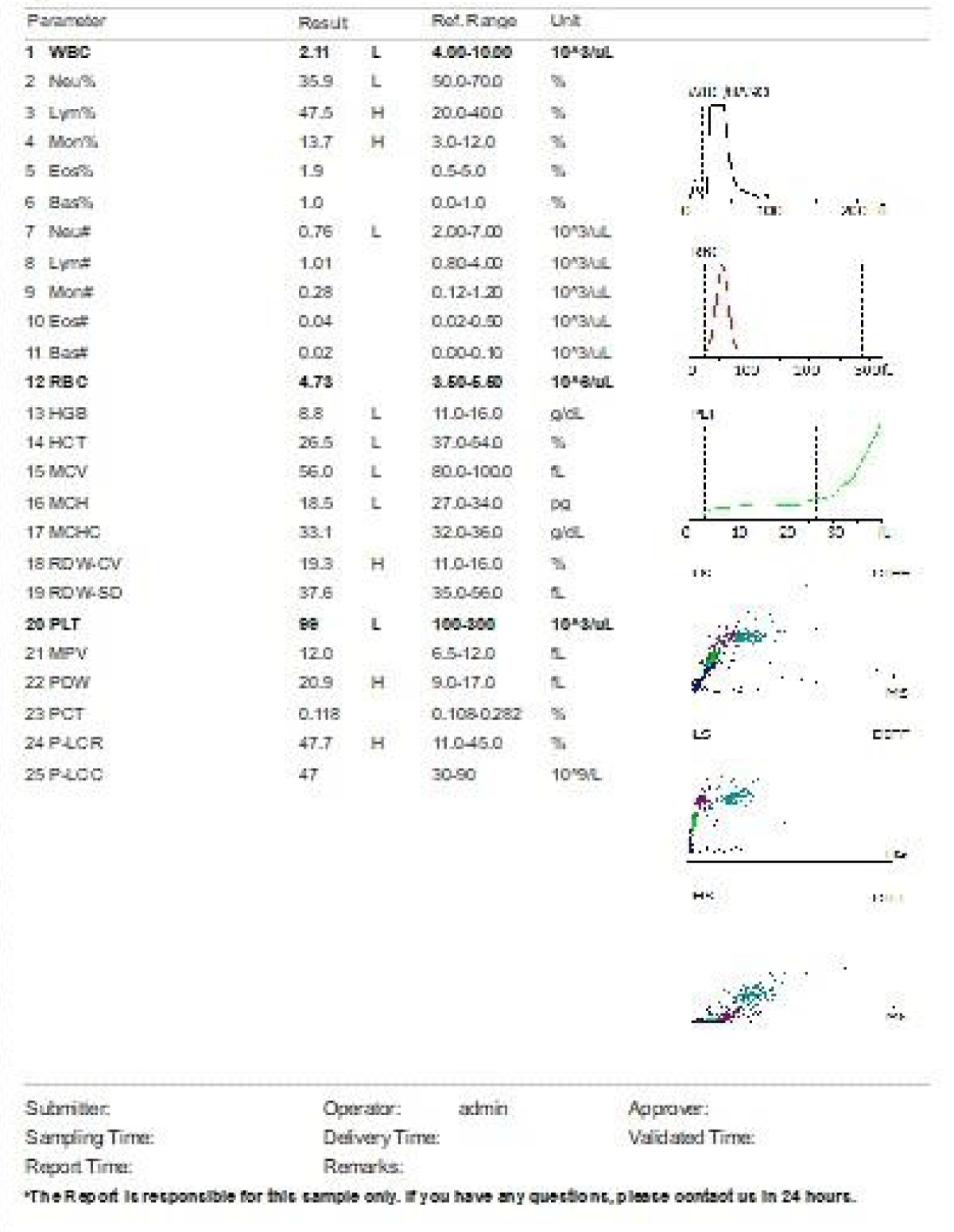
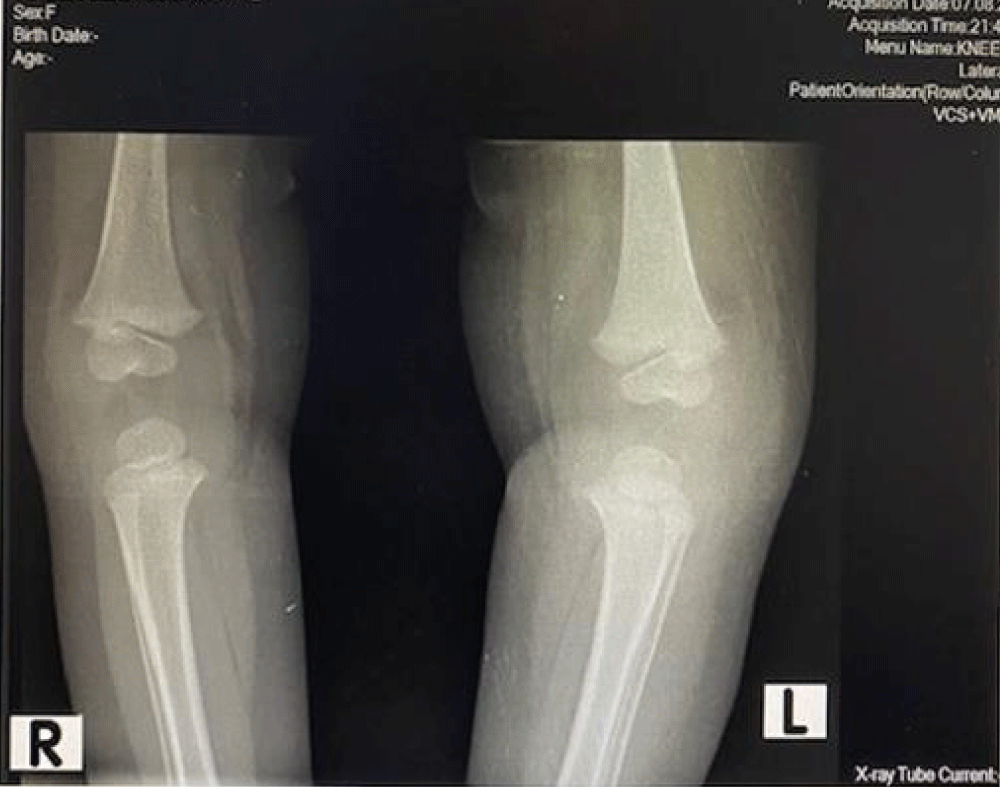
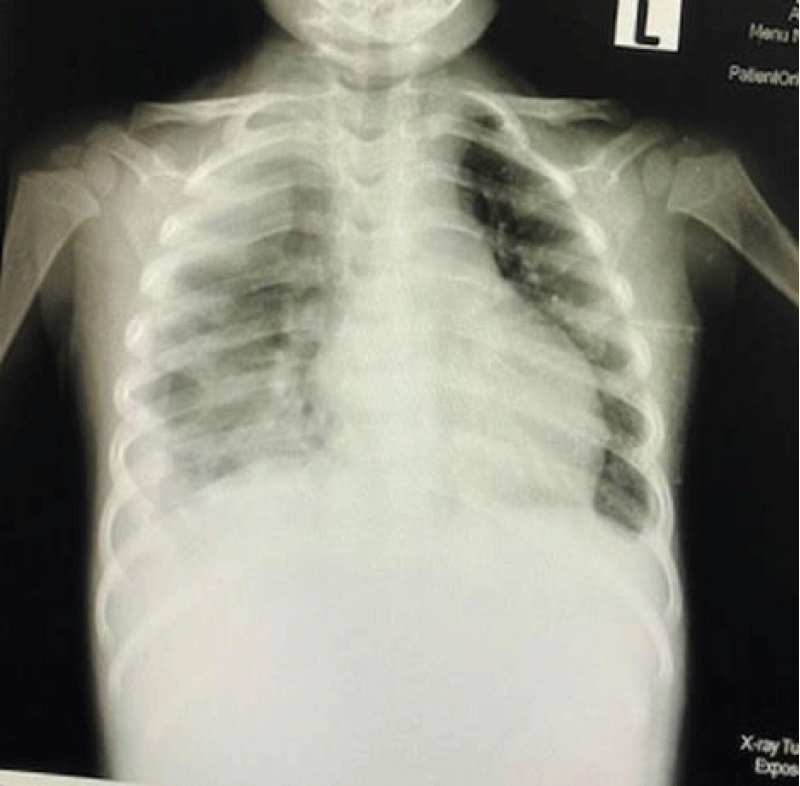
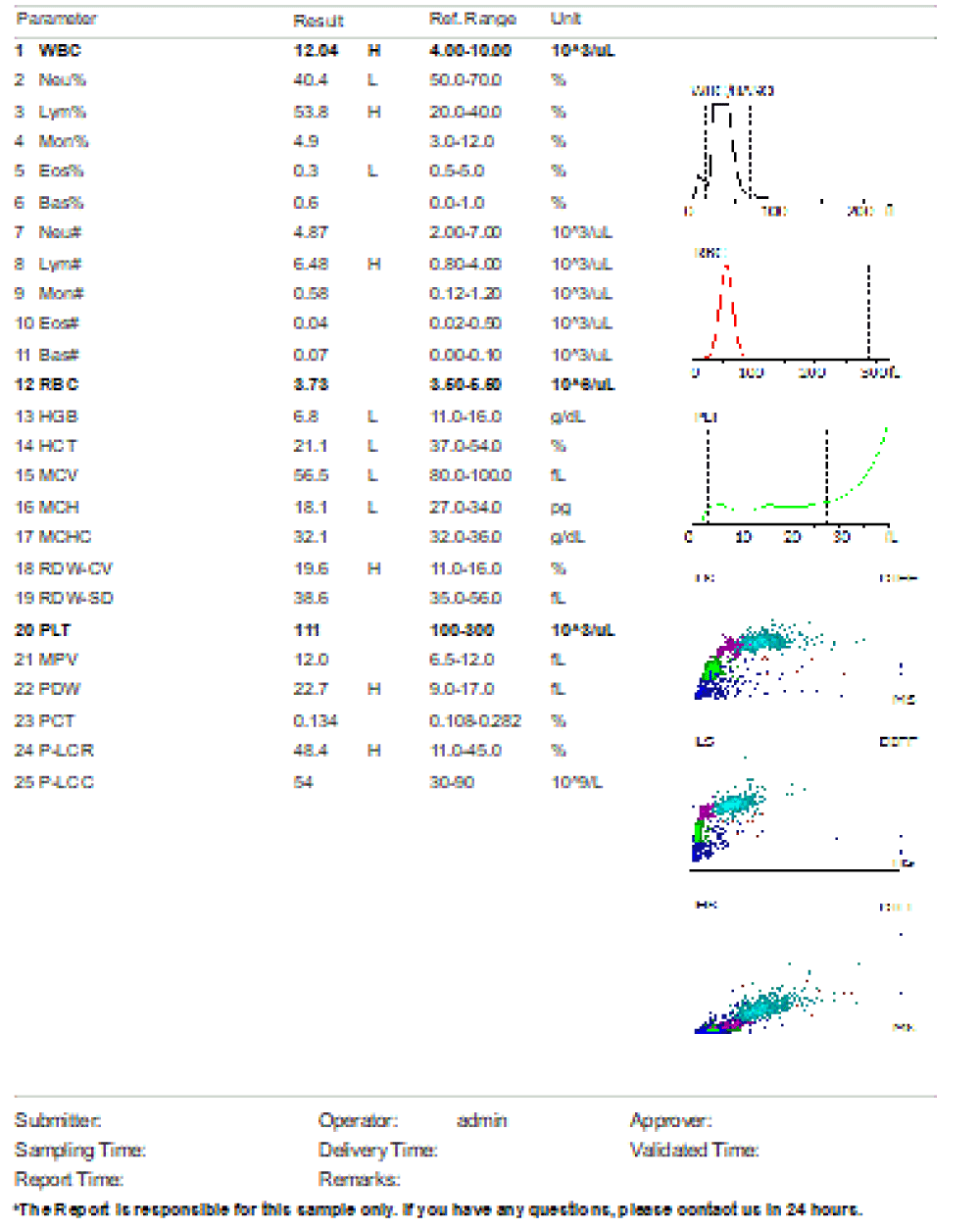
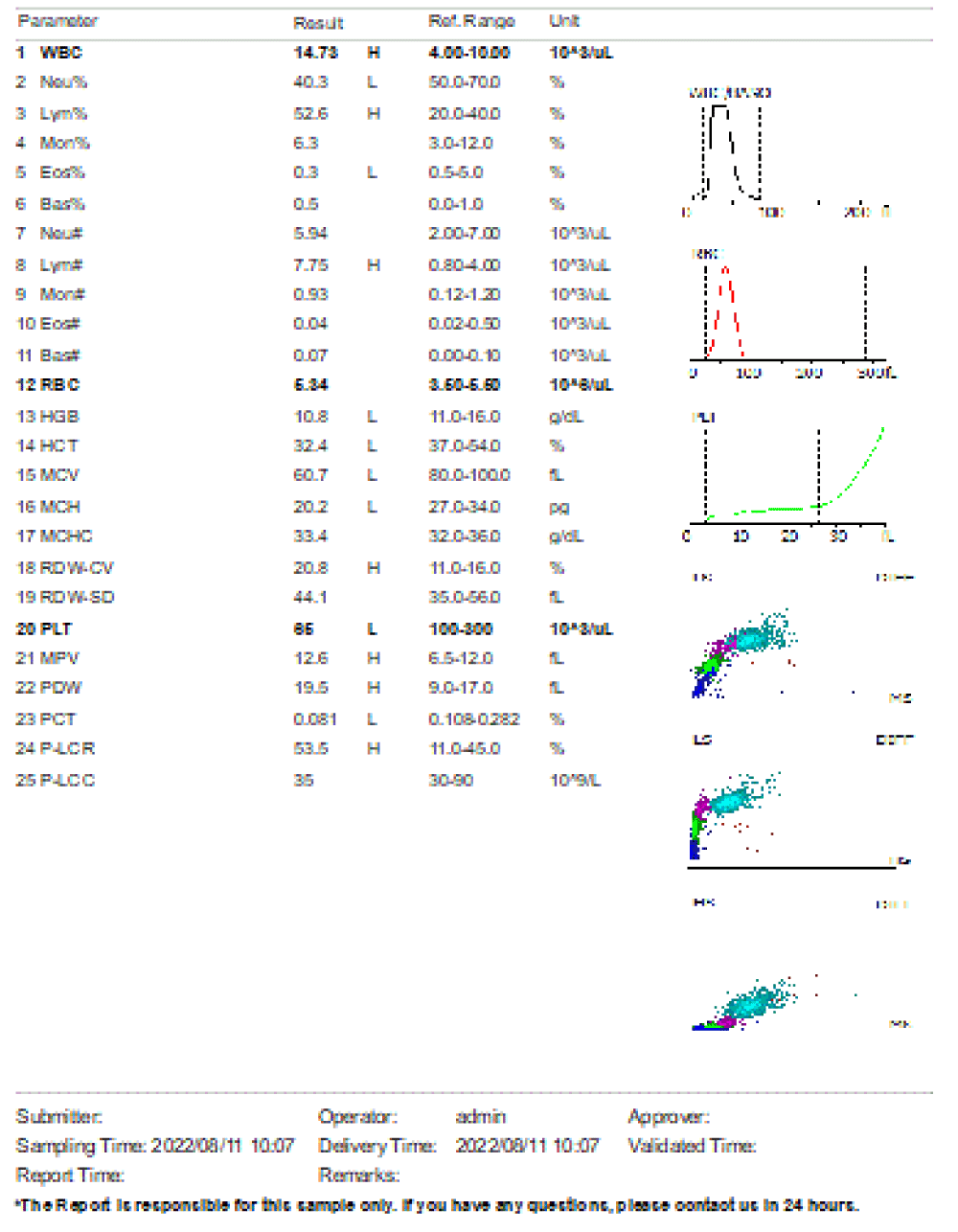
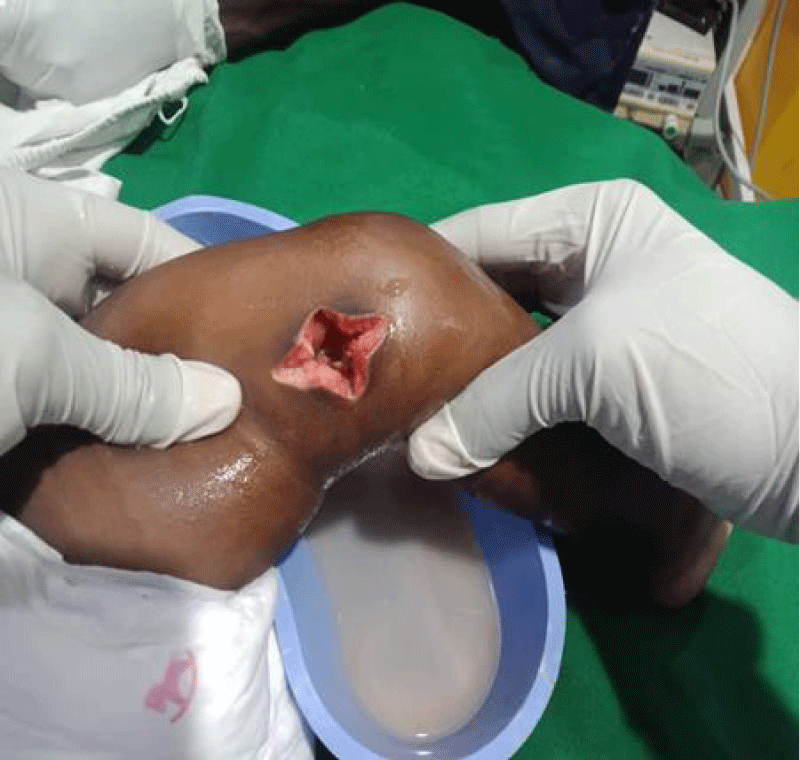
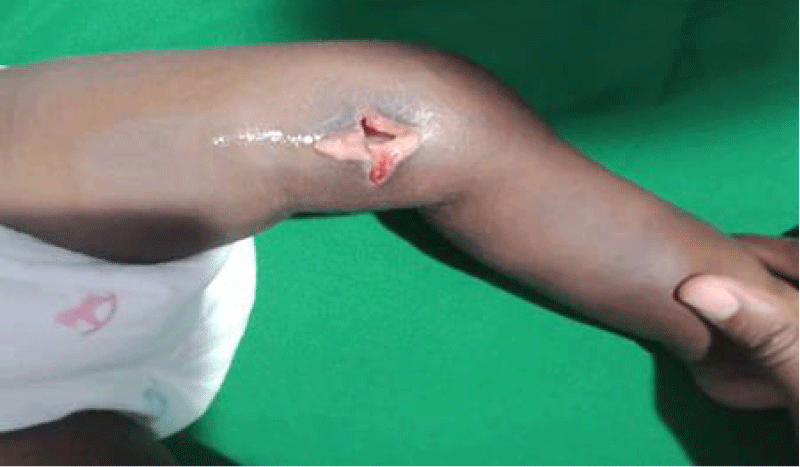
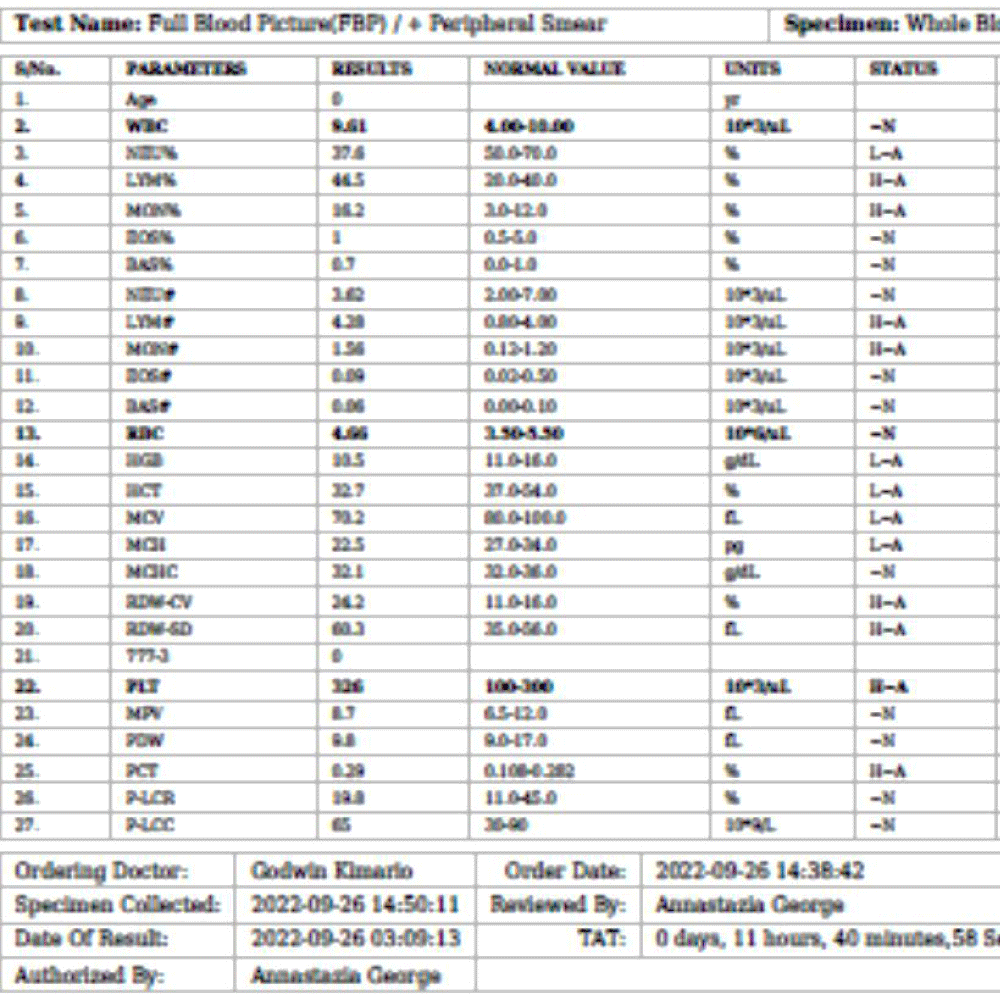
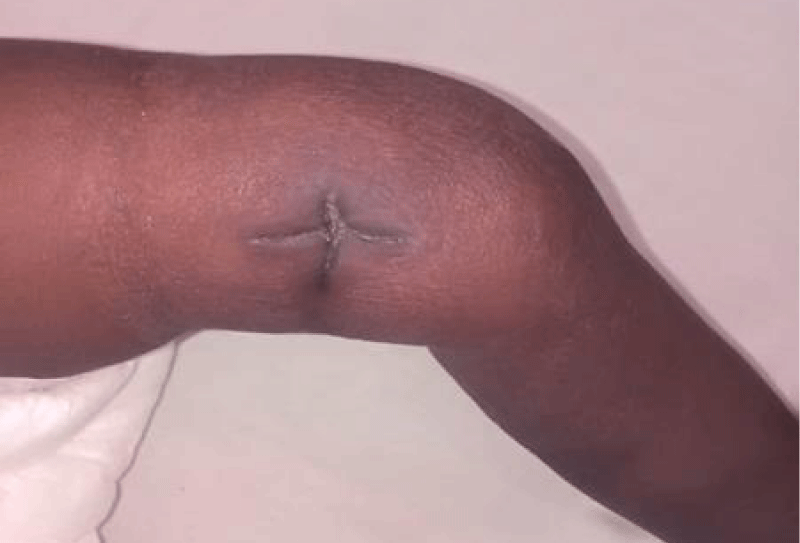
We are presenting a case of a 14-month-old African Tanzanian female who presented at our hospital with left knee pain and swelling that extended to the lower one-third of the thigh for three days. In addition, she also had diarrhea and abdominal pain for about four days. She suffered three motions per day of loose mucoid stool which was foul-smelling. The symptoms were accompanied by fever and anorexia. She was given ampiclox and analgesics before admission with no improvement. On examination, her weight was 9 kilograms with a height of 83 cm, weight for height z- score of -2 SD, alert, in pain, febrile 38°C, weak and pale. Unable to bear weight on the left leg and the left knee was in a flexed internally rotated position with a visibly swollen left knee joint and lower one-third of the thigh (Figure 1). It was tender to touch with a reduced range of movement, inflamed and warm. The right leg examination was normal. The initial provisional diagnosis included septic arthritis, amoebiasis, moderate anemia, moderate malnutrition, and with differential diagnosis of cellulitis, reactive arthritis, human immunodeficiency virus infection, and sickle cell disease. The complete blood count depicted neutropenia of 0.76 x 103 /UL, a hemoglobin level of 8.8 g/dl, a mean corpuscular volume of 56.0 fL, a mean corpuscular hemoglobin concentration of 33.1 g/dL, and platelets of 103 x 103/UL (Figure 2). The random blood glucose was 5.5 mmol/l and Entamoeba histolytica cysts were detected in the stool. Haemoglobin electrophoresis was done with HbAA detected ruling out sickle cell disease. A rapid HIV antibody test was non-reactive both for the child and the mother. X-ray of the left knee (Figure 3) showed a flexed and rotated left knee joint with bulging of the peri-articular fat pads without significant joint space widening nor juxta-articular osteopenia. A correlative ultrasound of the left knee showed hypoechoic intra-articular free fluid in the left knee joint demonstrating echogenic debris with marked periarticular soft tissue fat stranding with increased vascularity on the surrounding tissues suggestive of an ongoing infective/inflammatory process or arthritis. Blood culture and sensitivity samples were taken however, the results came back with no bacterial growth. The general surgeon and orthopedic surgeon were called to review the case and the patient was then initiated on amoxiclav, metronidazole, and acetaminophen and she kept on maintenance intravenous fluid. Arthrocentensis of the left knee joint was done with a clear straw color synovial fluid aspirated of which culture came back with no bacterial growth and gram stain showed white blood cell proliferation. The following day her condition deteriorated with episodes of high-grade fever, an increase in pain, and swelling of the left knee. A decision was made to change antibiotics to intravenous ceftriaxone and gentamycin. Over the night she developed difficulty in breathing with her oxygen saturation dropping to 70% on ambient air and was kept on oxygen therapy via face mask 10L/min with an improvement of 98% measured by pulse oximeter. On systemic examination, had tachypnea (respiratory rate 62 breaths per minute) with vesicular breath sounds. Mother also reported that she had vomited blood once over the night and on examination she had scleral jaundice. Acute liver failure and septic embolism were suspected secondary to the septic arthritis. She was transferred to the intensive care unit (ICU) and further investigations were done. Chest x-ray, allowing for rotation to the right, showed a segmental heterogeneous airspace opacification obscuring the right cardiac border suggestive of right middle lobe consolidation (Figure 4). Liver function tests were elevated with an aspartate transaminase level of 2046U/L, hypoalbuminemia of 2.2 mg/dl, total bilirubin was elevated to 9.21 mg/dl with direct bilirubin 8.3 mg/dl. Control complete blood count (CBC) showed leukocytosis and the haemoglobin level had dropped to 6.8 g/dl (Figure 5). Arterial blood gases were not available at the facility at that moment. Still, we had a high index of suspicion of metabolic acidosis and an empirical stat intravenous dose of sodium bicarbonate was given. Erythrocyte sedimentation rate and C-reactive protein were elevated at 20 mm/Hr and 9.5 mg/dl respectively. The echocardiogram and abdominal ultrasound were normal. Multiple episodes of hypoglycemia were recorded and resuscitation with Dextrose 10% and then Dextrose 5% Normal Saline (DNS) alternating with ringer lactate as a maintenance fluid regimen. She was transfused 180mls of whole blood and since platelets dropped the next day to 65 x 103 /UL (Figure 6) with visible oral mucosal and gum bleeding an additional transfusion of platelets 128mls and 135mls of fresh frozen plasma were given. Vancomycin was initiated unfortunately she had a hypersensitivity reaction developing generalized body swelling and therefore it was stopped. Meropenem, ciprofloxacin, and metronidazole were initiated with hoping of covering fastidious organisms such as Kingella kingae, salmonella, methicillin-resistant Staphylococcus aureus (MRSA), and gram-negative bacteria. Metronidazole was maintained to eradicate Entamoeba histolytica. Since biochemistry investigation revealed hypoalbuminemia, high liver enzymes (Aspartate transferase and Alanine Aminotransferase), hypoglycemia, high total bilirubin with high direct bilirubin and thrombocytopenia in CBC, an additional diagnosis of acute liver failure secondary to sepsis was confirmed. Prothrombin time/ International normalized ratio and partial thromboplastin time were unfortunately unavailable. Therefore, in addition to her prior management, she received dexamethasone, tranexamic acid, albumin, and kept nil per oral. Daily vitamin K injections were also given for three days. The following day she improved with her haemoglobin level rising to 10.8 g/dl and platelets to 111 x 103/UL after the transfusions. She stayed in the ICU for a total of five days and was transferred to the pediatric ward when her liver enzymes, bilirubin, and glucose levels normalized, and she was clinically stable. After 2 days in the ward, her left knee swelling became loculated and fluctuant. A second arthrocentesis revealed pus and an incision and drainage arthrotomy of the pyogenic arthritis was indicated. Under general anesthesia whereby a right knee distal thigh swelling 6 x6 cm fluctuant and shiny was examined followed by a sterile procedure medial aspect of the left knee joint cruciate incision arthrotomy and a gush of about 350mls of pus was drained which extended to the knee intra capsule with capsule of the opened and irrigated with normal saline one liter then with pivodone iodine and wound covered with gauze and supported with crepe bandage (Figures 7,8). A pus sample was taken for culture and gram staining for fastidious organisms, anaerobic, aerobic gram-positive, and gram-negative aerobic bacteria. After seven days all results showed no bacterial growth and gram stain showing an infiltration of white blood cells. Her complete blood count still showed leukocytosis and a low haemoglobin count of 8.5 g/dl but the reticulocyte count was within the normal range. In addition, her CRP dropped to 4.2 mg/dl after three weeks on intravenous antibiotics. She was then initiated oral clindamycin and had daily drainage and dressing with physiotherapy initially done under sedation until she improved. The pus decreased and after 3 weeks of clindamycin her hemoglobin level on follow up 9.5 g/dl with leucocyte of 12 x 103/L. At her follow-up clinic her complete blood count had returned to normal apart from the mild anemia of 10.5 g/dl (Figure 9) of which she was kept on iron therapy and her CRP was within normal range. The family was counseled on dietary requirements in addressing moderate malnutrition with high calorie, protein content with fruits and vegetables to address micronutrients and vitamins. She was in addition given multivitamins. Her follow-up plan included a bi-weekly visit for nutrition, physiotherapy, and assessment by both pediatrician and orthopedic. She progressed well and was expected to make a full recovery (Figure 10).
Figure 1: Left knee on the first day of presentation with flexion seen and swelling of the joint and distal thigh.
Figure 2: Day 1 on admission, complete blood count prior initiation of antibiotic. Leucopenia, neutropenia, moderate anemia and mild thrombocytopenia noted.
Figure 3: X-ray of the left knee showed a flexed and rotated left knee joint with bulging of the peri-articular fat pads without significant joint space widening nor juxta-articular osteopenia.
Figure 4: Chest x-ray, allowing for rotation to the right, showed a segmental heterogeneous airspace opacification obscuring the right cardiac border suggestive of right middle lobe consolidation.
Figure 5: Day 3 post admission, complete blood count with leukocytosis, severe anemia. Patient admitted in ICU for management and given blood transfusion 180mls of whole blood.
Figure 6: Day 5 post admission and Day 2 in ICU, developed thrombocytopenia with gum bleeding noted. Patient received platelets and FFP transfusion.
Figure 7: After sterile draping the leg was cleansed and under general anethesia a medial aspect of the left knee joint cruciate incision arthrotomy done a gush of about 350mls of pus was drained which extended to the knee intra capsule with capsule of the opened and irrigated with normal saline one liter then with pivodone iodine. Followed by the wound being covered with gauze and supported with crepe bandage.
Figure 8: Daily drainage, irrigation with normal saline, dressing and physiotherapy was done under general anesthesia for about four days with reduction in pus with dressing with gauze and crepe bandage.
Figure 9: Day 52 post admission, Day 10 post discharge follow up. Complete blood count shows mild anemia with resolving of thrombocytopenia and leukocytosis.
Figure 10: The incision wound on day ten post drainage with healed with joint swelling resolved.
The empirical management of septic arthritis is first-generation cephalosporin such as cephalexin, cefadroxil, cefazolin, or clindamycin [12]. A prevalence of MRSA of more than 10% prompts clindamycin as the first drug of choice taking into account that resistance to clindamycin is less than 10% if high then Vancomycin should be used although it has poor bone penetration [13]. Fastidious organism Kingella kingae is sensitive to cephalosporins and penicillin [14]. While Salmonella spp can be treated with third-generation cephalosporin or fluoroquinolones [15,16]. Our patient was initially started on ampicillin-cloxacillin and later to amoxicillin clavulanic acid with a suspicion of Staphylococcus aureus as the most common organism in our setting then ceftriaxone after 24 hours of no response to address Salmonella spp together with gentamycin was added. When MRSA was suspected vancomycin was started but she had an adverse reaction thence the meropenem, ciprofloxacin, and metronidazole (triple therapy) regimen. These organisms were considered but none were cultured only Entamoeba histolytica was confirmed in stool. Her poor response to multiple antibiotics led to the triple therapy which showed a response. She was maintained in the triple therapy for about three weeks and once able to take it orally, oral Clindamycin medication was initiated for an additional three weeks.
Fastidious organisms such as Kingella kingae are usually detectable by DNA polymerase chain reaction [17] which was unavailable at our facility. Kingella kingae has become a known causative agent, especially in children less than the age of three years old [18]. Isolating the organism is 5.6 times more sensitive from throat cultures than synovial fluid [19]. Thus a consideration for throat swab cultures can be done in children presenting with septic arthritis for detection of Kingella kingae. It is usually sensitive to penicillin, fluoroquinolones, and trimethoprim-sulfamethoxazole but resistance has been reported [17]. An algorithm for the prediction of Kingella kingae infection includes a body temperature less than 38 °C, leucocyte counts less than 14,000/UL with bands less than 150/mm, and a C-reactive protein of less than 5.5 mg/dl [20]. Therefore such cases are mostly mild as compared with our patient which might suggest that it was unlikely Kingea kingae infection but cannot be ruled out.
In about 50% of synovial fluid culture results turn out negative [21] implying culture should not be the core investigation to rule out septic arthritis. In another study of 129 pediatric cases with septic arthritis, only 31% of culture came positive and in only 24.8% it was detectable in synovial fluid [22]. Such erratic nature in the detection of organisms in culture prompted the usage of criteria indices for the diagnosis of septic arthritis. The diagnosis of septic arthritis includes the presence of a tender inflamed joint, with a child’s refusal to bear weight on it, fever, a raised estimated sedimentation rate greater than 20mm/Hr, or C- reactive protein of greater than 2.0 mg/dl. Our patient met the criteria for a high suspicion index for septic arthritis. A white blood cell count of more than 50000/µL in the synovial fluid is highly suggestive of septic arthritis [23].
C-reactive protein(CRP) is preferred in monitoring treatment response than ESR, CRP levels usually return to normal within a week in treatment-responding patients while ESR might take up to months to fall to normal ranges [24,25]. Our patient’s CRP started to improve when she was initiated the triple therapy and maintained on clindamycin until it fell back to normal range (Figure 5). Apart from the difficulty in the detection of the organism via culture, gram staining also posed a similar trend. Gram staining of the synovial fluid is crucial but also difficult since the fluid has bacteriostatic characteristics making it difficult to culture organisms and about 50% of results are sterile even in those with positive blood culture results or other suggestive laboratory findings [4,26-28]. Which was also observed with our patient.
Reactive arthritis was a differential diagnosis since the patient had presented with gastrointestinal symptoms namely diarrhea and abdominal pain with Entamoeba histolytica cysts in the stool. Reactive arthritis, is a form of arthritis seen when there other systemic infections preceding the arthritis with no organisms detected in culture or gram staining either through blood or synovial fluid sample [29]. Organisms that have been identified to cause reactive arthritis are Yersinia, Shigella, Chlamydia trachomatis, Escherichia coli, Chlamydia pneumoniae, Campylobacter, Clostridium difficile and Salmonella [30-32]. The World Health Organization in collaboration with the Arthritis and Rheumatic Research Council has grouped reactive arthritis into four categories namely reactive arthritis with infectious or septic arthritis with the offending organism identified in the joint secondary to an infection elsewhere in the body, reactive arthritis post-infectious with the organism antigens detectable in joint, reactive arthritis of urogenital or gastrointestinal origin leading to inflammatory joint reaction but the organism is not detected in the joint and reactive arthritis which is triggered by an infection of which neither the organism, it’s antigen nor products are detectable in the joint [33]. Recently an update in the list of the organisms that can cause reactive arthritis has been added namely gastrointestinal organisms including amoebae, Cryptosporidium, Giardia lamblia, and helminths Strongyloides spp. Also respiratory, urogenital, and miscellaneous organisms including Human immunodeficiency virus (HIV), Borrellia burgdorferi, Parvovirus B19, Chikungunya virus, and Calmette-Guerin Bacillus [34]. Reactive arthritis mostly occurs a couple of weeks after an infection and mostly affects adults. Our patient’s knee symptoms occurred in conjunction with her gastrointestinal symptoms which can occur [35] and the lack of the classical triad namely conjunctivitis, urethritis, and arthritis did not support reactive arthritis although this has been reported in some cases [36]. If she had an atypical presentation of reactive arthritis our case falls in the category where the reactive arthritis occurred with gastrointestinal symptoms and the systemic organism was identified in stool as Entamoeba histolytica but was not detectable in the joint. The presence of sepsis with acute liver failure and pus from the joint supported septic arthritis rather than reactive arthritis and therefore it was ruled out.
The mortality rate in septic arthritis is high although recent medical advancements and approaches to diagnosing septic arthritis have reduced the mortality rate. Mortality has been reported to range from 4% to 42% [37-39] thus it requires an emergency intervention since invading organisms cause joint cartilage damage which if not corrected can result in permanent damage to the joint and bone. Infection of the joint results from hematogenous spread, penetrating wounds, invasive surgical procedures, or spreading from a bone infection. A study done in Nigeria identified hematogenous route and penetrating wound as the most common cause of septic arthritis in more than 90% of children [40].
Hematogenous spread toward the joint is favorable due to the capillary blood supply in the joint is sluggish and the absence of the basement membrane in the synovium predisposes the seeding of the bacteria via the haematogenous route. Once the microorganism seeding ensues a cascade of events follows which trigger inflammation and signaling of macrophages, cytokines, and proteases. The influx causes a buildup of pressure in the joint manifesting as pain and thus limiting the range of motion. The cytotoxic agents released by the bacteria coupling with the inflammatory process foster cartilage invasion [41]. At this stage further white blood cell pooling into the joint causes the release of protease and cytokines leading to the destruction of chondrocytes and inhibiting cartilage synthesis. The increase of intra-articular pressure predisposes to vascular thrombosis the endpoint of which is ischemia and this further destroys the cartilage and leads to bone erosion [41,42]. This damage can occur as early as forty-eight hours after the joint is infected and therefore septic arthritis is regarded as a surgical emergency. In the index case treatment was initiated within an hour upon arrival with intravenous antibiotics and surgical debridement with incision and drainage via open arthrotomy was done on the fourth day. Open arthrotomy, irrigation, antibiotics both intravenous and oral with physiotherapy are the management deployed to ensure she made full recovery.
Several risk factors have been reported to be associated with septic arthritis. In Nigeria, they identified sickle cell disease, malnutrition, and HIV as predisposing factors [40]. Our patient weighed 8.5 kg and height of 83 cm with weight for height z score -2 at the age of 14 months hence moderately wasted. Sickle cell disease was ruled out by Hb electrophoresis and rapid HIV antibody test results were negative.
Septic arthritis can complicate and result in anemia, septic shock, joint stiffness, pathologic dislocation if the hip is involved, and osteonecrosis [40]. Our patient had anemia that required transfusion and septic shock with acute liver failure. The anemia is linked to hemolysis and bone marrow toxic effect of sepsis [43]. She still has some stiffness in the left knee joint and is ongoing rehabilitative physiotherapy.
Septic arthritis is a serious condition and requires early identification and treatment. The disease is mostly severe in children less than the age of five years with a haematogenous route from peripheral foci as a cause. Infections from the respiratory, gastrointestinal, and urinary tracts can result in septic arthritis with causative organisms including aerobic gram-positive and gram-negative, fastidious organisms such as Kingella kingae. Empirical antibiotics initiation, surgical drainage, and rehabilitative physiotherapy are crucial to ensure healing and joint functionality are maintained.
Contributors
GB: manuscript writing, patient management, SY, JS patient management mainly microbiology and manuscript revision, RM, MI, FM, FS: manuscript revision and patient management, RK: manuscript editing, patient management; PN: manuscript editing and imaging, JS: patient management mainly microbiology and manuscript revision. All authors approved the final version of this manuscript and are responsible for all aspects of this study.
- Krogstad P. Osteomyelitis and septic arthritis In: Feigin RD, Cherry JD, editors Textbook of Pediatric Infectious Diseases 6th ed Philadelphia, PA: Saunders; 2009; 725–748.
- Lavy CB, Peek AC, Manjolo G. The incidence of septic arthritis in Malawian children. Int Orthop. 2005 Jun;29(3):195-6. doi: 10.1007/s00264-005-0643-9. Epub 2005 Apr 2. PMID: 15806359; PMCID: PMC3456879.
- Nunn TR, Cheung WY, Rollinson PD. A prospective study of pyogenic sepsis of the hip in childhood. J Bone Joint Surg Br. 2007 Jan;89(1):100-6. doi: 10.1302/0301-620X.89B1.17940. PMID: 17259425.
- Welkon CJ, Long SS, Fisher MC, Alburger PD. Pyogenic arthritis in infants and children: a review of 95 cases. Pediatr Infect Dis. 1986 Nov-Dec;5(6):669-76. doi: 10.1097/00006454-198611000-00014. PMID: 3540888.
- Castellazzi L, Mantero M, Esposito S. Update on the Management of Pediatric Acute Osteomyelitis and Septic Arthritis. Int J Mol Sci. 2016 Jun 1;17(6):855. doi: 10.3390/ijms17060855. PMID: 27258258; PMCID: PMC4926389.
- Khattak M, Vellathussery Chakkalakumbil S, Stevenson RA, Bryson DJ, Reidy MJ, Talbot CL, et al. Kingella kingae septic arthritis. Bone Joint J. 2021 Mar;103-B(3):584-8.
- Yagupsky P. Changing aetiology of paediatric septic arthritis. J Paediatr Child Health. 2021 Oct;57(10):1560-1563. doi: 10.1111/jpc.15654. Epub 2021 Jul 14. PMID: 34259365.
- al-Salem AH, Ahmed HA, Qaisaruddin S, al-Jam'a A, Elbashier AM, al-Dabbous I. Osteomyelitis and septic arthritis in sickle cell disease in the eastern province of Saudi Arabia. Int Orthop. 1992;16(4):398-402. doi: 10.1007/BF00189627. PMID: 1473897.Nduati RW, Wamola IA Bacteriology of acute septic arthritis Journal of tropical pediatrics 1991 Aug 1;37(4):172-5.
- Lavy CB, Lavy VR, Anderson I. Salmonella septic arthritis of the shoulder in Zambian children. J R Coll Surg Edinb. 1996 Jun;41(3):197-9. PMID: 8763188.
- Molyneux E, French G. Salmonella joint infection in Malawian children. J Infect. 1982 Mar;4(2):131-8. doi: 10.1016/s0163-4453(82)93701-x. PMID: 6764502.
- Peltola H, Pääkkönen M, Kallio P, Kallio MJ; OM-SA Study Group. Clindamycin vs. first-generation cephalosporins for acute osteoarticular infections of childhood--a prospective quasi-randomized controlled trial. Clin Microbiol Infect. 2012 Jun;18(6):582-9. doi: 10.1111/j.1469-0691.2011.03643.x. Epub 2011 Oct 19. PMID: 22011265.
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF; Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011 Feb 1;52(3):e18-55. doi: 10.1093/cid/ciq146. Epub 2011 Jan 4. Erratum in: Clin Infect Dis. 2011 Aug 1;53(3):319. PMID: 21208910.
- Saphyakhajon P, Joshi AY, Huskins WC, Henry NK, Boyce TG. Empiric antibiotic therapy for acute osteoarticular infections with suspected methicillin-resistant Staphylococcus aureus or Kingella. Pediatr Infect Dis J. 2008 Aug;27(8):765-7. doi: 10.1097/INF.0b013e31816fc34c. PMID: 18600193.
- Sherman JW, Conte JE Jr. Ceftriaxone treatment of multidrug-resistant Salmonella osteomyelitis. Am J Med. 1987 Jul;83(1):137-8. doi: 10.1016/0002-9343(87)90508-0. PMID: 3605165.
- Bradley JS, Jackson MA; Committee on Infectious Diseases; American Academy of Pediatrics. The use of systemic and topical fluoroquinolones. Pediatrics. 2011 Oct;128(4):e1034-45. doi: 10.1542/peds.2011-1496. Epub 2011 Sep 26. PMID: 21949152.
- Yagupsky P, Porsch E, St Geme JW 3rd. Kingella kingae: an emerging pathogen in young children. Pediatrics. 2011 Mar;127(3):557-65. doi: 10.1542/peds.2010-1867. Epub 2011 Feb 14. PMID: 21321033.
- Ilharreborde B, Bidet P, Lorrot M, Even J, Mariani-Kurkdjian P, Liguori S, Vitoux C, Lefevre Y, Doit C, Fitoussi F, Penneçot G, Bingen E, Mazda K, Bonacorsi S. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J Clin Microbiol. 2009 Jun;47(6):1837-41. doi: 10.1128/JCM.00144-09. Epub 2009 Apr 15. Erratum in: J Clin Microbiol. 2009 Sep;47(9):3071. PMID: 19369442; PMCID: PMC2691089.
- Basmaci R, Ilharreborde B, Bidet P, Doit C, Lorrot M, Mazda K, Bingen E, Bonacorsi S. Isolation of Kingella kingae in the oropharynx during K. kingae arthritis in children. Clin Microbiol Infect. 2012 May;18(5):E134-6. doi: 10.1111/j.1469-0691.2012.03799.x. Epub 2012 Mar 6. PMID: 22390653.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013 Feb;25(1):58-63. doi: 10.1097/MOP.0b013e32835c2b42. PMID: 23283291.
- Russell CD, Ramaesh R, Kalima P, Murray A, Gaston MS. Microbiological characteristics of acute osteoarticular infections in children. J Med Microbiol. 2015 Apr;64(Pt 4):446-453. doi: 10.1099/jmm.0.000026. Epub 2015 Jan 16. PMID: 25596125.
- Spyridakis E, Gerber JS, Schriver E, Grundmeier RW, Porsch EA, St Geme JW, Downes KJ. Clinical Features and Outcomes of Children with Culture-Negative Septic Arthritis. J Pediatric Infect Dis Soc. 2019 Jul 1;8(3):228-234. doi: 10.1093/jpids/piy034. PMID: 29718310.
- Pääkkönen M, Kallio MJ, Kallio PE, Peltola H. Sensitivity of erythrocyte sedimentation rate and C-reactive protein in childhood bone and joint infections. Clin Orthop Relat Res. 2010 Mar;468(3):861-6. doi: 10.1007/s11999-009-0936-1. Epub 2009 Jun 17. PMID: 19533263; PMCID: PMC2816763.
- Stans AA. Osteomyelitis and septic arthritis In: Lovell and Winter's Pediatric Orthopaedics, 6th ed, Morrissy RT, Weinstein SL (Eds), Lippincott Williams & Wilkins, Philadelphia. 2006; 440.
- Kallio MJ, Unkila-Kallio L, Aalto K, Peltola H. Serum C-reactive protein, erythrocyte sedimentation rate and white blood cell count in septic arthritis of children. Pediatr Infect Dis J. 1997 Apr;16(4):411-3. doi: 10.1097/00006454-199704000-00015. PMID: 9109146.
- Kallio MJ, Unkila-Kallio L, Aalto K, Peltola H. Serum C-reactive protein, erythrocyte sedimentation rate and white blood cell count in septic arthritis of children. Pediatr Infect Dis J. 1997 Apr;16(4):411-3. doi: 10.1097/00006454-199704000-00015. PMID: 9109146.
- Wiley JJ, Fraser GA. Septic arthritis in childhood. Can J Surg. 1979 Jul;22(4):326-30. PMID: 313236.
- Nade S, Robertson FW, Taylor TK. Antibiotics in the treatment of acute osteomyelitis and acute septic arthritis in children. Med J Aust. 1974 Nov 9;2(19):703-5. doi: 10.5694/j.1326-5377.1974.tb71104.x. PMID: 4444617.
- Ahvonen P, Sievers K, Aho K. Arthritis associated with Yersinia enterocolitica infection. Acta Rheumatol Scand. 1969;15(3):232-53. doi: 10.3109/rhe1.1969.15.issue-1-4.32. PMID: 4902690.
- Rohekar S, Pope J. Epidemiologic approaches to infection and immunity: the case of reactive arthritis. Curr Opin Rheumatol. 2009 Jul;21(4):386-90. doi: 10.1097/BOR.0b013e32832aac66. PMID: 19373091.
- Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum. 2007 Aug;37(1):48-55. doi: 10.1016/j.semarthrit.2006.12.006. Epub 2007 Mar 13. PMID: 17360026; PMCID: PMC2909271.
- Coath FL, Mackay J, Gaffney JK. Axial presentation of reactive arthritis secondary to COVID-19 infection. Rheumatology (Oxford). 2021 Jul 1;60(7):e232-e233. doi: 10.1093/rheumatology/keab009. PMID: 33471106; PMCID: PMC7928576.
- Mathew AJ, Ravindran V. Infections and arthritis. Best Pract Res Clin Rheumatol. 2014 Dec;28(6):935-59. doi: 10.1016/j.berh.2015.04.009. Epub 2015 May 27. PMID: 26096095.
- García-Kutzbach A, Chacón-Súchite J, García-Ferrer H, Iraheta I. Reactive arthritis: update 2018. Clin Rheumatol. 2018 Apr;37(4):869-874. doi: 10.1007/s10067-018-4022-5. Epub 2018 Feb 17. PMID: 29455267.
- Piussan C, Pautard-Muchemble B, Pautard JC, Lenaerts C, Boudailliez B, Risbourg B. Les arthrites réactionnelles de l'enfant [Reactive arthritis in children]. Sem Hop. 1984 Jan 12;60(1):52-62. French. PMID: 6320436.
- Arnett FC. Incomplete Reiter's syndrome: clinical comparisons with classical triad. Ann Rheum Dis. 1979;38 Suppl 1(Suppl 1):suppl 73-8. doi: 10.1136/ard.38.suppl_1.73. PMID: 318103; PMCID: PMC1049089.
- Coakley G, Mathews C, Field M, Jones A, Kingsley G, Walker D, Phillips M, Bradish C, McLachlan A, Mohammed R, Weston V; British Society for Rheumatology Standards, Guidelines and Audit Working Group. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology (Oxford). 2006 Aug;45(8):1039-41. doi: 10.1093/rheumatology/kel163a. Epub 2006 Jul 6. PMID: 16829534.
- Kaandorp CJ, Krijnen P, Moens HJ, Habbema JD, van Schaardenburg D. The outcome of bacterial arthritis: a prospective community-based study. Arthritis Rheum. 1997 May;40(5):884-92. doi: 10.1002/art.1780400516. PMID: 9153550.
- Fangtham M, Baer AN. Methicillin-resistant Staphylococcus aureus arthritis in adults: case report and review of the literature. Semin Arthritis Rheum. 2012 Feb;41(4):604-10. doi: 10.1016/j.semarthrit.2011.06.018. Epub 2011 Oct 28. PMID: 22035623.
- Omoke NI, Obasi AA. Childhood Pyogenic Septic Arthritis as Seen in a Teaching Hospital South East Nigeria. Niger J Surg. 2017 Jan-Jun;23(1):26-32. doi: 10.4103/1117-6806.199968. PMID: 28584508; PMCID: PMC5441212.
- Kendal J. Septic Arthritis: Pathogenesis and clinical findings. 2014. wwwcalgaryguidecom.
- McCarthy JJ, Dormans JP, Kozin SH, Pizzutillo PD. Musculoskeletal infections in children: basic treatment principles and recent advancements. Instr Course Lect. 2005;54:515-28. PMID: 15948476.
- Akinyoola AL, Obiajunwa PO, Oginni LM. Septic arthritis in children. West Afr J Med. 2006 Apr-Jun;25(2):119-23. doi: 10.4314/wajm.v25i2.28260. PMID: 16918182.